-
上转换发光(即反斯托克斯发光)是用波长较长的光激发样品,发射出波长小于激发光波长的光的现象[1], 即用小能量光子激发而得到大能量光子发射的现象。AUZEL和PISARSKA等人[2-3]对稀土离子掺杂的上转换特性和机制进行系统研究后,提出由激发态吸收、能量传递以及合作敏化引起的上转换发光过程。稀土上转换发光材料可用于上转换激光器[4]、防伪技术[5-6]、新型显示器[7]等,未来将在很多领域得到广泛应用。
稀土元素掺杂ZnO材料,可同时利用稀土离子4f-4f跃迁和ZnO独特的半导体性能。这种结构的材料有利于上转换发光,因而得到广泛研究。ISHIZUMI等人[8]采用微乳液法制备出ZnO:Eu纳米棒,得出Eu3+上转换发光效率主要由其激发态能量弛豫过程决定的结论。LÜ等人[9]通过化学沉淀法制备出ZnO:Er3+粉体, 在室温下观测到上转换发光现象。ZHANG等人[10]采用煅烧法制备出ZnO:Dy粉体,并对其结构和光学性能进行分析,得出粉体的激发谱和发射谱是由激发波长和Dy3+浓度决定的结论。WU等人[11]通过高温氧化法制备出Yb3+/Er3+共掺ZnO粉体,在488nm和980nm两种激光激发下都观察到Er3+上转换发光现象。MIGUEL等人[12]采用熔体淬火法制备出Yb3+/Er3+共掺氟碲酸盐玻璃,得出红绿上转换发光取决于Yb3+浓度的结论。CHENG等人[13]由高温固相法制备出CaWO4:Yb3+ / Er3+粉体,确定上转换发光过程及荧光强度参量。XIAO等人[6]通过ZnF2分解法制备出ZnO:Er3+粉体,在980nm激光激发下观测到红绿上转换发光强度比为21:1。发射的红光强度远大于绿光强度,ZnO中稀土离子间团聚效应是产生强红光的主要原因[11, 14]。Yb3+和Er3+共掺ZnF2粉末上转换发光是否也有这种现象,值得探讨和研究。
与ZnO基质相比,氟化物基质具有声子能量低、无辐射跃迁损失小的特点[15-19],具有较高上转换效率。加之敏化剂Yb3+与激活离子Er3+之间可发生有效能量传递,从而使上转换发光明显增强[20-22]。但是,目前对于Yb3+和Er3+共掺ZnF2粉末上转换发光特性的研究鲜见报道。
本文中主要研究ZnF2作为基质材料,Yb3+,Er3+共掺摩尔分数不同时,所制备样品的上转换发光性能。对制备的各个样品进行上转换发射光谱测试,并将激发功率与上转换发射功率进行曲线拟合,确定Yb3+和Er3+光子吸收过程。
-
每一份样品中,ZnF2(化学纯)基质都准确称量15g。掺杂Er2O3摩尔分数x分别为0.002, 0.005,Yb2O3摩尔分数分别为x, 2x, 4x, 8x。NH4F中F-用以替代稀土氧化物中O2-,按化学计量比6:1计算得到,替代量为计算结果的1.5倍。并对各个样品进行编号,如表 1所示。
Table 1. Mole fraction of Er2O3-Yb2O3 in each sample
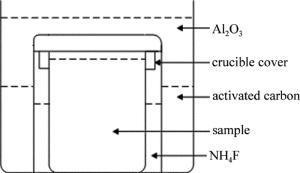
sample number 1# 2# 3# 4# 5# 6# 7# 8# Er2O3 0.002 0.002 0.002 0.002 0.005 0.005 0.005 0.005 Yb2O3 0.002 0.004 0.008 0.016 0.005 0.010 0.020 0.040 按照摩尔分数关系,分别计算出各个样品所需Er2O3,Yb2O3和NH4F具体含量。准确称量原料,充分研磨并混合均匀。按照顺序依次放入8个如图 1所示的制备装置中,并做标记1#~8#。将各个样品都放入硅碳棒马弗炉中加热到820℃,并保持恒温4h,立即取出并快速冷却。冷却后的样品进行研磨,分别装入8个样品袋,准备测试光谱特性。
如图 1所示,Al2O3粉末质地细密,该层可以很好地隔绝空气;活性炭层主要用于吸收氧气;NH4F高温分解为NH3和HF,可提供氟化氢气氛,同时也可作为助熔剂[23]。
-
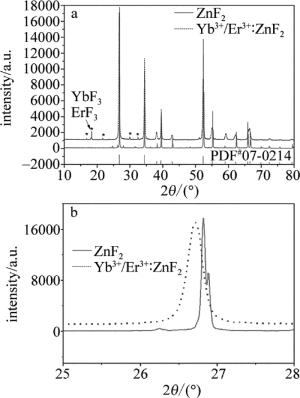
样品物相组成分析,采用的仪器是BRUKER D8 focus型X射线衍射仪,Cu靶X射线源(波长λ=0.15418nm),可扫描范围是10°~80°。制备样品的X射线衍射(X-ray diffraction,XRD)图谱(Er2O3的摩尔分数为0.005, Yb2O3的摩尔分数为0.020)如图 2所示。图 2a中实线表示ZnF2衍射图谱,虚线表示Yb3+/Er3+共掺ZnF2衍射图谱,星号表示YbF3和ErF3特征峰。图 2a中PDF#07-0214为ZnF2标准便携式文件格式(portable document format,PDF)卡片衍射图谱。需要说明的是,其余掺杂比例样品XRD特征峰分布也和此一致,图中未列出。图 2b是扫描区间25°~28°的放大图。
由图 2a可看到,粉末材料主要成分为ZnF2,有少量YbF3和ErF3生成。意味着样品制备过程中发生反应[23](Yb2O3+HFYbF3+H2O;Er2O3+HFErF3+H2O)。图 2b中实线表示ZnF2衍射图谱,虚线表示Yb3+/Er3+共掺ZnF2衍射图谱。布喇格公式如下:
$ 2d\text{sin}\theta =n\lambda $
(1) 式中,d表示晶面间距,θ表示掠射角(也称布喇格角,范围为0°~90°),n表示衍射级数,λ表示X射线波长。在n和λ一定时,θ越小,d越大。由图 2b可得,Yb3+/Er3+共掺ZnF2衍射峰左移,掠射角θ变小,晶面间距d变大。由于Yb3+和Er3+离子半径比Zn2+离子半径大,所以掠射角θ变小说明Yb3+和Er3+已掺入ZnF2晶格中。Yb3+作为敏化剂,Er3+作为激活剂。
-
样品上转换发射谱数据,是由Zolix Omi-λ150型单色仪和PMTH-S1-CR131型光电倍增管测量得到。稀土上转换材料发射谱测试装置系统框图,如图 3所示。
稀土上转换材料发射谱测试,在密闭的暗箱中进行。980nm半导体激光器(laser diode,LD)作为激发光源,照射到置于样品架上的待测样品;待测样品受激发射产生上转换光,被收集到单色仪入射狭缝;经过单色仪分光,不同波长的上转换光从单色仪出射狭缝入射到光电倍增管内,经过光电倍增管转换后的电信号值被计算机记录。
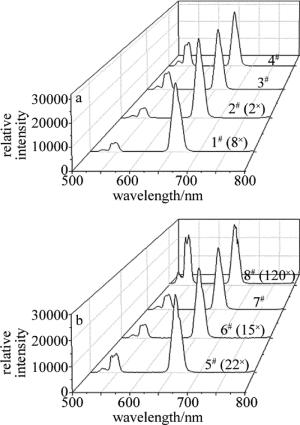
每个样品上转换发射谱数据,都是在同等条件下测试完成。因而,各发射峰强度具有可比性。为便于分析比较,将各样品光谱图进行缩放处理,结果如图 4所示。
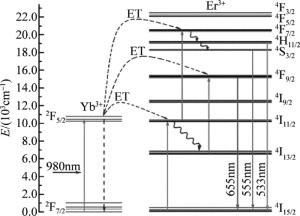
由图 4可知,在980nm LD激发下,所得产物在可见光范围内存在两个中心波长分别位于533nm, 555nm附近的绿光带和一个中心波长位于655nm附近的红光带。通过对照Er3+能级结构简图,确定跃迁过程分别对应于Er3+的2H11/2→4I15/2, 4S3/2→4I15/2和4F9/2→4I15/2辐射跃迁。Yb3+和Er3+之间能量传递(energy transfer,ET)示意图和可能上转换过程,如图 5所示。
在以上的上转换发光中,Er3+可直接吸收一个光子跃迁至4I11/2能级。处于4I11/2能级的Er3+可继续吸收一个光子,跃迁至4F7/2能级。即:
$ ^{4}{{\text{I}}_{11/2}}(\text{E}{{\text{r}}^{3+}})+h\nu {{\to }^{4}}{{\text{F}}_{7/2}}(\text{E}{{\text{r}}^{3+}}) $
(2) 式中,hν表示光子能量,h是普朗克常数,ν是光波的频率。
或者两个Er3+之间发生交叉弛豫能量转移,即:
$ \begin{align} & ^{4}{{\text{I}}_{11/2}}\left( \text{E}{{\text{r}}^{3+}} \right){{+}^{4}}{{\text{I}}_{11/2}}\left( \text{E}{{\text{r}}^{3+}} \right)\to ~ \\ & ^{4}{{\text{F}}_{7/2}}\left( \text{E}{{\text{r}}^{3+}} \right){{+}^{4}}{{\text{I}}_{15/2}}\left( \text{E}{{\text{r}}^{3+}} \right)~ \\ \end{align} $
(3) 由于Yb3+的2F5/2能级在980nm处有较高的吸收截面,与Er3+的4I11/2能级相匹配,Yb3+-Er3+对之间有着高效率的能量传递。通过逐次能量转移上转换发光机制,Er3+可吸收Yb3+处于激发态能级2F5/2的能量,从而实现Er3+的4F7/2能级粒子数布居,即:
$ \begin{align} & ^{2}{{\text{F}}_{5/2}}(\text{Y}{{\text{b}}^{3+}}){{+}^{4}}{{\text{I}}_{11/2}}(\text{E}{{\text{r}}^{3+}})\to ~ \\ & ^{2}{{\text{F}}_{7/2}}(\text{Y}{{\text{b}}^{3+}}){{+}^{4}}{{\text{F}}_{7/2}}(\text{E}{{\text{r}}^{3+}})~ \\ \end{align} $
(4) 处于4F7/2能级的Er3+通过无辐射弛豫跃迁至2H11/2和4S3/2能级,然后分别向4I15/2能级跃迁,依次发射出中心波长为533nm和555nm的绿光。处于4I11/2能级的大部分Er3+由多声子弛豫现象,无辐射跃迁至4I13/2能级。进而跃迁至4F9/2能级,实现粒子数布居。处于4F9/2能级的Er3+跃迁至基态4I15/2能级,发射出中心波长为655nm的红光。
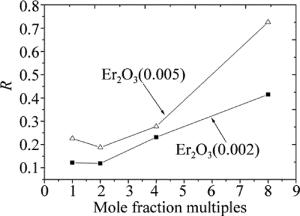
在实际应用中,通过改变稀土离子共掺杂比例来影响材料样品的上转换发光性能,进行调色。对于每个样品发射谱数据,中心波长为533nm和555nm两个发射峰的总积分面积记为Sg,中心波长为655nm发射峰的积分面积记为Sr。取比值,即R=Sg/Sr。不同样品上转换发射谱红绿光强度比值(横坐标表示Yb2O3与Er2O3摩尔分数倍数),如图 6所示。
通常情况下,在Yb3+,Er3+共掺上转换发光材料中,发射的绿光强度要大于红光强度,实际观察的现象却与此相反。WU[11]和OFELT[14]等人研究认为,稀土离子间团聚效应引起的交叉弛豫能量转移过程应该是产生强红光的主要原因。考虑到Er3+间交叉能量传递过程[24]:
$ ^{4}{{\text{I}}_{11/2}}\left( \text{E}{{\text{r}}^{3+}} \right){{+}^{4}}{{\text{F}}_{7/2}}\left( \text{E}{{\text{r}}^{3+}} \right)\to {{2}^{4}}{{\text{F}}_{9/2}}\left( \text{E}{{\text{r}}^{3+}} \right)~ $
(5) $ \begin{align} &{{\text{ }\!\!~\!\!\text{ }}^{\text{4}}}{{\text{I}}_{\text{15/2}}}\left( \text{E}{{\text{r}}^{\text{3+}}} \right){{\text{+}}^{\text{2}}}{{\text{H}}_{\text{11/2}}}{{\text{/}}^{\text{4}}}{{\text{S}}_{\text{3/2}}}\left( \text{E}{{\text{r}}^{\text{3+}}} \right)\to \\ &^{\text{4}}{{\text{I}}_{\text{13/2}}}\left( \text{E}{{\text{r}}^{\text{3+}}} \right){{\text{+}}^{\text{4}}}{{\text{I}}_{\text{9/2}}}\left( \text{E}{{\text{r}}^{\text{3+}}} \right)\text{ }\!\!~\!\!\text{ } \\ \end{align} $
(6) $ ^{\text{4}}{{\text{I}}_{\text{15/2}}}\left( \text{E}{{\text{r}}^{\text{3+}}} \right){{\text{+}}^{\text{4}}}{{\text{I}}_{\text{9/2}}}\left( \text{E}{{\text{r}}^{\text{3+}}} \right)\to {{\text{2}}^{\text{4}}}{{\text{I}}_{\text{13/2}}}\text{(E}{{\text{r}}^{\text{3+}}}\text{)} $
(7) $ \begin{align} &^{\text{2}}{{\text{F}}_{\text{5/2}}}\left( \text{Y}{{\text{b}}^{\text{3+}}} \right){{\text{+}}^{\text{4}}}{{\text{I}}_{\text{13/2}}}\left( \text{E}{{\text{r}}^{\text{3+}}} \right)\to ~ \\ &^{\text{2}}{{\text{F}}_{\text{7/2}}}\left( \text{Y}{{\text{b}}^{\text{3+}}} \right){{\text{+}}^{\text{4}}}{{\text{F}}_{\text{9/2}}}\left( \text{E}{{\text{r}}^{\text{3+}}} \right)\text{ }\!\!~\!\!\text{ } \\ \end{align} $
(8) 处于2H11/2,4S3/2能级粒子数减少和(7)式所示过程,使得4I13/2能级粒子数急剧增加。(8)式所示过程发生概率增大,加之由于4I13/2能级具有较长寿命[25],处于4I13/2能级的Er3+易于跃迁至4F9/2能级。总之,这些上转换过程均有利于Er3+能级4F9/2粒子数布居。因而由图 6可观察到,所得产物发射的红光强度要大于绿光强度。
当Yb2O3与Er2O3摩尔分数倍数较小时,R值变化缓慢;当Er3+摩尔分数一定时,随着加到ZnF2基质中Yb3+比例的逐渐增加,(4)式所示过程在加强,从而引起绿光强度增加。随着Yb3+比例继续增加,上转换发光可能出现饱和现象,Er3+向Yb3+反向能量传递增强[11, 26]。处于4F7/2能级处的Er3+急剧减少,进而使得2H11/2和4S3/2能级粒子数布居减少,但对于Er3+之间交叉弛豫能量转移过程影响较小。总而言之,随着Yb3+比例增加,R值在增大。
-
在可使用功率范围内,激光器输出功率与其工作电流呈线性关系,用下式表述,即:
$ ~{{P}_{\text{out}}}=k(i-{{i}_{0}}) $
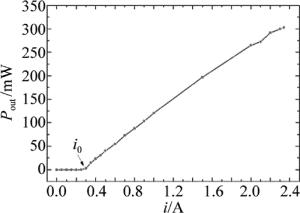
(9) 式中,k是与激光器工作特性相关的比例常数,i是激光器工作电流,i0是激光器阈值电流。
由图 7可知,实验中使用的980nm LD存在阈值电流i0。当激光器工作电流小于i0时,无激光输出;当激光器工作电流大于i0时,输出功率与工作电流呈线性关系。稀土上转换材料发光功率Pupcon与激光器输出功率Pout的关系式为[27]:
$ {{P}_{\text{upcon}}}=~{{P}_{\text{out}}}^{m} $
(10) 式中,m表示吸收光子数目。比如说,m=2表示双光子吸收,m=3表示三光子吸收等等。
联立(9)式和(10)式得:
$ {{P}_{\text{upcon}}}={{k}^{m}}{{(i-{{i}_{0}})}^{m}} $
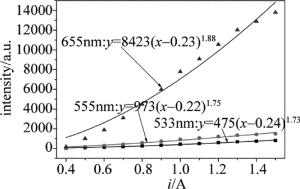
(11) 样品上转换发射功率与激光器工作电流关系的拟合曲线,如图 8所示。图中,点表示实际所测数据,3条曲线表示经过拟合后的结果。所对应的m值分别为1.73,1.75和1.88,都接近于2。可以看出,上转换发射的533nm, 555nm和655nm这3个波长的发光均对应于双光子吸收。
-
研究了ZnF2作为基质材料,稀土离子Yb3+和Er3+共掺摩尔分数不同时,所制备样品的上转换发光性能。在820℃时制备样品,所得产物主要为ZnF2和少量YbF3, ErF3。在980nm LD激发下,可见光区域内观察到533nm, 555nm和655nm 3个波长的发射光。由样品发射功率随激光器工作电流变化的拟合曲线,得到吸收光子数目依次为1.73, 1.75, 1.88,确定3个波长的发光均为双光子吸收。发射的红光强度要大于绿光强度,稀土离子间团聚效应引起的交叉弛豫能量转移过程应该是产生强红光的主要原因。研究结果表明, 稀土离子掺杂ZnF2材料,将在上转换红色荧光粉领域有重要的应用前景。
Yb3+/Er3+共掺ZnF2粉末上转换发光特性研究
Study on up-conversion luminescence properties of Yb3+/Er3+ co-doped ZnF2 powder
-
摘要: 为了研究ZnF2作为基质材料、稀土离子Yb3+和Er3+共掺摩尔分数不同时的发光性能,采用高温固相法,在820℃时制备稀土掺杂ZnF2样品,并对各个样品进行上转换发射光谱测试。将激发功率与上转换发射功率进行曲线拟合,确定Yb3+和Er3+光子吸收过程。结果表明,在980nm半导体激光器激发下,样品在可见光区域内存在533nm,555nm和655nm 3个上转换发射峰,发射的红光强度大于绿光强度,吸收光子数目依次为1.73,1.75,1.88,确定3个发射峰均对应于双光子吸收。此研究说明稀土离子掺杂ZnF2材料将在上转换红色荧光粉领域有重要的应用前景。Abstract: In order to study luminescent properties of rare earth ions Yb3+ and Er3+ with different co-doped molar fractions and ZnF2 as matrix material, by using high temperature solid state method, samples of rare earth doped by ZnF2 at 820℃ were prepared and the up-conversion emission spectra was measured. Yb3+ and Er3+ photon absorption processes were determined by fitting the excitation power and up-conversion power. The results show that under the excitation of 980nm laser diodes, the samples have 3 up-conversion emission peaks of 533nm, 555nm and 655nm in the visible region. The intensity of red light is greater than that of green light.The number of the absorbed photons is 1.73, 1.75, and 1.88. 3 emission peaks are correspond to two-photon absorption.The materials of rare earth ion doped by ZnF2 have important application prospects in up-conversion red phosphors.
-
Table 1. Mole fraction of Er2O3-Yb2O3 in each sample
sample number 1# 2# 3# 4# 5# 6# 7# 8# Er2O3 0.002 0.002 0.002 0.002 0.005 0.005 0.005 0.005 Yb2O3 0.002 0.004 0.008 0.016 0.005 0.010 0.020 0.040 -
[1] TIAN J, LUO H F. Research development of up-converting rare earth nanophosphors [J]. Journal of Chemical Education, 2014, 35(20):1-4 (in Chinese). [2] AUZEL F E. Materials and devices using double-pumped phosphors with energy-transfer [J]. Proceedings of the IEEE, 1973, 61(6):758-786. doi: 10.1109/PROC.1973.9155 [3] WRIGHT J C, ZALUCHA D J, LAUER H V, et al. Up-conversion and excited state energy transfer in rare-earth doped materials [J]. Journal Applied Physics, 1973, 44(2):781-789. doi: 10.1063/1.1662260 [4] WANG F, BANERJEE D, LIU Y S, et al. Up-conversion nanoparticles in biological labeling, imaging and therapy [J]. Analyst, 2010, 135(8):1839-1854. doi: 10.1039/c0an00144a [5] WANG W. Research and application of rare earth up-conversion luminescent materials [J]. Guangzhou Chemical Industry, 2014, 42(11):32-34(in Chinese). [6] XIAO S G, YANG X L, LIU Z W, et al. Strong red up-conversion in Er3+ doped zinc oxide powder prepared by fluoride salt decomposition method [J].Optical Materials, 2006, 28(3):285-288. doi: 10.1016/j.optmat.2004.12.016 [7] HONG G Y. Research progress of rare earth luminescent materials [J]. Journal of Synthetic Crystals, 2015, 44(10):2641-2651(in Chinese). [8] ISHIZUMI A, KANERMITSU Y. Structural and luminescence properties of Eu-doped ZnO nanorods fabricated by a microemulsion method [J].Applied Physics Letters, 2005, 86(25):253106. doi: 10.1063/1.1952576 [9] LÜ Sh Ch, SONG G L, XIAO Zh Y, et al. Preparation and room temperature up-conversion luminescence of nanocrystalline ZnO:Er3+ by chemical precipitation [J]. Journal of the Chinese Rare Earth Society, 2002, 20(s2): 273-275 (in Chinese). [10] ZHANG L L, GUO C X, ZHAO J J, et al. Synthesis of ZnO:Dy nanopowder and photo luminescence of Dy3+ in ZnO [J].Journal of Rare Earths, 2005, 23(5):607-610. [11] WU Q, YANG L W, LIU Y X. Frequency up-conversion properties of Er3+/Yb3+ co-doped zinc oxide powders [J]. Spectroscopy and Spectral Analysis, 2008, 28(7): 1473-1478(in Chinese). [12] MIGUEL A, ARRIANDIAGA M A, MOREA R, et al. Down- and up-conversion emissions in Er3+-Yb3+ co-doped TeO2-ZnO-ZnF2 glasses [J]. Journal of Luminescence, 2015, 158(2):142-148. [13] CHENG X R, YANG K, WANG J K, et al. Up-conversion luminescence and optical temperature sensing behaviour of Yb3+/Er3+ co-doped CaWO4 material [J]. Optical Materials, 2016, 58(8):449-453. [14] OFELT G S. Intensities of crystal spectra of rare-earth ions [J].Journal of Chemical Physics, 1962, 37(3):511-520. doi: 10.1063/1.1701366 [15] ZHANG R R, GAO Y, TANG B. Lanthanide-doped fluoride nanoparticles: up-conversion luminescence and biological applications [J].Journal of Analytical Science, 2010, 26(3):353-357(in Chinese). [16] CHEN G Y, OHULCHANSKYY T Y, KACHYNSKI A V, et al. Intense visible and near-infrared up-conversion photo luminescence in colloidal LiYF4:Er3+ nanocrystals under excitation at 1490nm [J]. American Chemical Society Nano, 2011, 5(6):4981-4986. [17] SUYVER J F, GRIMM J, VEEN M K, et al.Up-conversion spectroscopy and properties of NaYF4 doped with Er3+, Tm3+ and/or Yb3+ [J]. Journal of Luminescence, 2006, 117(1):1-12. doi: 10.1016/j.jlumin.2005.03.011 [18] IVANOVA S E, PELLE F, TKACHU K, et al. Up-conversion luminescence dynamics of Er-doped fluoride crystals for optical converters [J]. Journal of Luminescence, 2008, 128(5):914-917. [19] JIA Y J, LIN J, ZHANG W J. Effect of fluoride on up-conversion and infrared luminescence properties of Er3+/ Yb3+ co-doped tellurite glass [J]. Chinese Journal of Luminescence, 2014, 35(3):287-292 (in Chinese). doi: 10.3788/fgxb [20] MAO Y L, LIN B Ch, FENG S Y, et al. The spectroscopic property of erbium ytterbium co-doped a luminamaterial prepared by sol-gel process [J]. Laser Technology, 2007, 31(6):600-606(in Chin-ese). [21] LI B Ch, WANG H B, ZHUO N Z. The rare earth up-conversion luminescent materials overview [J].China Light & Lighting, 2015(6):10-14(in Chinese). [22] ZHANG X Y, GAO D L, LI L, et al. Factors affecting fluorescent efficiency of frequency up-conversion of rare earth [J]. Laser Technology, 2010, 34(6):855-860 (in Chinese). [23] HONG G Y. Fundamental and application of rare earth luminescent materials [M]. Beijing: Science Press, 2011:451(in Chinese) [24] PU W, JUDITH M, DAWES P B. Diode-pumped cw tunable Er3+:Yb3+:YCOB laser at 1.5-1.6μm [J]. Optical Materials, 2002, 19(3):383-387. doi: 10.1016/S0925-3467(01)00241-5 [25] FRORENZO V, JOHN C B, JOHN A C. Significance of Yb3+ concentration on the up-conversion mechanisms in codoped Y2O3:Er3+, Yb3+ nanocrystals [J].Journal of Applied Physics, 2004, 96(1):661-667. doi: 10.1063/1.1739523 [26] HOU Y B, CHEN X B, ZHANG G Y. Energy transfer in up-conversion luminescence of Pr, Yb:ZBLAN [J]. Acta Optica Sinica, 1997, 17(4):403-408 (in Chinese). [27] POLLNAU M, GAMELIN D R, LUTHI S R, et al. Power dependence of up-conversion luminescence in lanthanide and transition metalion systems[J]. Physical Review, 2000, B61(5):3337-3346. -

 网站地图
网站地图

 下载:
下载: