Research status of Ti-6Al-4V manufactured by selective laser melting for medical device applications
-
摘要: 激光选区熔化技术(SLM)因其具备高复杂结构制造能力而在医疗器械制造上展现着独特的优势。近年来,SLM在医疗器械制造上日趋活跃,综述了基于激光选区熔化成形、应用于创伤外科医疗器械生产制造Ti-6Al-4V的研究现状。包括SLM成形Ti-6Al-4V的基础工艺、力学性能、细胞相容性三方面的研究进展,同时举例了一些基于SLM成形Ti-6Al-4V医疗器械的应用及案例。最后针对基于此技术制造医疗器械研究存在的问题进行了总结。尽管SLM成形Ti-6Al-4V在医疗领域发展相对缓慢,但现有的研究和潜力表明,随着基础制造研究的不断深入和临床不断的实践,创伤外科的精准治疗方案将无疑有望成为可能。Abstract: Selective laser melting (SLM) is used in medical device manufacturing recently due to the unique capability of fabricating high-complexity structures. With the growing popularity of SLM in medical device manufacturing, this paper mainly reviews the research progress of Ti-6Al-4V fabricated by SLM for surgical medical device applications, including the basic technics of SLM-Ti-6Al-4V fabrication, the achievable mechanical properties, cell compatibility, and some application cases. In addition, we summarizes the underlying problems in the field of medical device manufacturing based on the SLM tethnics. Although the development of SLM formed Ti-6Al-4V is relatively slow in the field of medical application, the existing research and potential indicate that with the continuous intensive research of basic manufacture and the continuous clinical practice, the precise treatment of trauma surgery will undoubtedly become possible.
-
Keywords:
- optical fabrication /
- selective laser melting /
- Ti-6Al-4V /
- medical devices
-
引言
碳纤维复合材料(carbon fiber reinforced plastic, CFRP)作为一种新型复合材料具有比强度高、低密度和抗疲劳性好等优异的力学性能,被广泛应用在航空航天、医疗设备、军工产品等[1]领域。在CFRP装配过程中,对CFRP进行开槽、钻孔等二次加工是不可避免的。CFRP常规的加工方法有铣削[2]、磨料水射流[3-4]、钻孔[5]、电火花加工[6]等。然而,CFRP具有复杂的各项异性且层间强度低,这些常规的加工方法加工CFRP时易出现材料分层、纤维断裂、毛刺和刀具磨损严重等缺陷[2, 7-8]。
与常规的加工方法相比,激光加工具有无刀具磨损、无接触应力等优点,被广泛用于材料加工。HEJJAJI等人[9]实施了常规钻孔和激光加工玻璃纤维复合材料和CFRP的实验研究,结果表明,激光加工效率更高。YE等人[10]对比了激光打孔和机械钻孔两种加工方式的特点,结果表明,两种加工方式下拉伸性能基本相近,但激光打孔具有更高的表面加工质量。但是激光加工CFRP容易造成较大的热影响区、纤维拔出和纤维末端膨胀等热损伤[11-12]。RIVEIRO等人[13]研究发现,虽然激光加工产生的热损伤是不可避免的,但是可以通过优化工艺参数使热损伤最小化。LEONE等人[14]使用波长1064 nm、平均功率为150 W的Nd∶YAG脉冲激光加工1 mm厚的CFRP板并优化了工艺参数,实验中热影响区(heat-affected zone, HAZ)低至170 μm。与此同时,发现在激光加工过程中增加辅助装置可以有效降低加工产生的热损伤。SOBRI等人[15]研究了辅助气体对激光加工CFRP的影响,结果表明,施加辅助气体有利于降低HAZ。
然而,辅助气体对降低HAZ有限。TANGWARODOMNUKUN等人[16]对比了激光在空气和水中的加工质量,结果表明,水辅助激光加工可以获得更小的热损伤。采用水辅助激光加工时,由于水对激光传输的影响大,不恰当的供液方式容易造成激光能量损失严重,导致加工效率下降[17]。WEE等人[18]在使用薄水层辅助激光加工时发现,当水层厚度超过某个临界值之后,将无法实现材料去除。因此,为了减少水对激光损失的影响,必须减薄流动水层厚度并提高水层流速。GUO等人[19]采用水雾辅助激光加工化学气相沉积金刚石时发现,水雾撞壁形成的流动水层薄且流速快,可以获得较好的加工质量,适用于辅助激光加工[20]。
综上所述,采用水雾辅助激光加工CFRP有望实现低损伤高效率加工的可能。但迄今为止,鲜有使用水雾辅助激光加工CFRP的报道;更重要的是,水雾参数对激光传输的影响尚未被实验研究。本文作者研究了CFRP的水雾辅助激光加工技术,采用光束分析仪和激光功率计研究了水雾对激光传输的影响规律;采用正交实验优化方法,得到了最优工艺参数组合;最后通过回归分析建立了工艺参数和加工质量之间的经验公式。该研究为激光低损伤加工CFRP提供了参考。
1. 材料、设备与方法
1.1 材料
本实验中使用的工件材料是CFRP单向层压板。CFRP厚度为1 mm,由异面正交状态的单层结构CFRP组成,共有14层,碳纤维丝束直径约为5 μm~10 μm,如图 1a和图 1b所示。碳纤维的体积分数为70%,树脂的体积分数为30%,CFRP样件的主要热物性参数如表 1所示。本文中使用截面HAZ(后文简称HAZ)、槽深和深宽比作为加工质量的评价指标,具体定义如图 1c所示,其中深宽比是指槽深与槽宽的比值,HAZ的数值取截面最大的HAZ。
表 1 CFRP的主要热物性参数Table 1. Physical properties of CFRPindex density/(kg·m-3) thermal conductively/(W·m-1·K-1) specific heat capacity/(J·kg-1·K-1) vaporization temperature/K latent heat/(kJ·kg-1) volume fraction/% epoxy resin 1200 0.1 1884 698 1000 30 carbon fiber 1850 50(axial); 5(radial) 710 3900 43000 70 1.2 设备
水雾辅助激光加工实验系统如图 2所示。系统使用的激光器为武汉荣科激光自动化有限公司生产的准连续光纤激光焊接/切割一体机(型号:WCM-IQCW450), 激光器采用IPG准连续光纤激光器(型号:YLM-450_4500-QCW), 准连续光纤激光的主要参数如表 2所示,能够在连续激光和脉冲激光模式下工作。
表 2 准连续激光器系统主要参数Table 2. Main parameters of quasi-continuous fiber laser systemparameter values parameter values wavelength/nm 1064 peak laser power/W 4500 pulse width/ms 0.1~50 focused spot radius/μm ≥50 single pulse energy/J ≤45 scanning speed/(mm·s-1) 0~200 工件被固定在XY运动平台上的载物台表面。水雾系统包括气源(氮气)、管道、流量调节阀、虹吸式喷嘴、回收容器和水箱。实验中采用氮气作为辅助气体,纯净水作为水源。高速流动的气体通过虹吸原理将水吸入到喷嘴中,在气体的作用下实现雾化。喷嘴以旁轴的形式将水雾射流喷射在CFRP表面,形成快速流动的薄水膜并流过激光消融区域以实现冷却效应。使用光束分析仪(测量光斑直径范围为22 μm~3.2 mm)测量不同条件下激光能量空间分布形态;使用激光功率计(最大测量激光功率50 W,检测波长范围19 nm~11 000 nm)测量不同条件下激光功率损失情况。使用测量激光显微镜观察烧蚀形貌并测量烧蚀数据。
1.3 方法
在本文中,水雾射流撞击CFRP壁面实际效果如图 3a所示。喷嘴高度h是指喷嘴出口中心到CFRP表面的投影距离,喷嘴倾角ϕ是指喷嘴中心线与水平方向的夹角(锐角),如图 3b所示,气体压力p是指辅助气体的表压。为了探索喷嘴高度、喷嘴倾角和气体压力变化时对激光加工CFRP的HAZ、槽深和深宽比的影响规律,设计了三因素四水平共16组正交实验对CFRP进行单次切割实验,实验参数如表 3所示。本实验中采用的激光工艺参数:激光功率为1800 W,激光频率为60 Hz,切割速率为3 mm/s,脉宽为0.1 ms。激光烧蚀点与水雾射流冲击点相距16 mm,如图 3b所示。为了降低实验误差,本实验中每个工艺参数将会重复进行4次,实验结果将取4次数据的平均值作为最终结果。同时与相同参数下的传统气体辅助激光切割质量进行对比。
表 3 正交实验参数配置表Table 3. Configuration of orthogonal experiment parametersfactors nozzle height/mm gas pressure/MPa nozzle angle/(°) level 1 10 0.2 20 level 2 20 0.3 30 level 3 30 0.4 40 level 4 40 0.5 50 2. 实验结果与讨论
2.1 水雾辅助激光加工CFRP正交实验结果分析
统计分析可以用来证明实验中控制因素在95%的置信水平上是显著的(表 4中表示不拒绝原假设程度的P值小于0.05)。本文中通过数理统计分析了实验因素中对各加工质量影响的主次顺序、显著性以及各实验因素的最优水平组合。研究表明,表 4中表示整个拟合方程显著性的F值越大,对加工质量的影响和贡献越大[21],R2为相关系数。
表 4 水雾辅助激光加工CFRP的方差分析Table 4. Variance analysis of water-mist assisted laser processing of CFRPresponse factor control factor degrees of freedom sum of squares mean square F P R2 cross section HAZ nozzle angle/(°) 3 23.543 7.848 0.614 0.630 0.781 gas pressure/MPa 3 109.129 36.376 2.848 0.127 nozzle height/mm 3 140.066 46.689 3.656 0.083 residual 6 76.633 12.772 total 15 groove depth nozzle angle/(°) 3 24174.156 8058.052 4.127 0.066 0.829 gas pressure/MPa 3 19038.062 6346.021 3.250 0.102 nozzle height/mm 6 13537.219 4512.406 2.311 0.176 residual 3 11714.750 1952.458 total 15 depth-width ratio nozzle angle/(°) 3 1.482 0.494 6.953 0.022 0.843 gas pressure/MPa 3 0.351 0.117 1.646 0.276 nozzle height/mm 3 0.449 0.150 2.106 0.201 residual 6 0.426 0.071 total 15 水雾参数对HAZ、槽深和深宽比的方差分析结果如表 4所示。统计分析中R2均大于75%,这表明了该结论是可信的。从表 4可以看出,对于HAZ而言,喷嘴高度具有一定的影响,其余因素都不具有显著影响;从图 4可以看出,当气体压力逐渐增加时,HAZ、槽深和深宽比均逐渐减小。原因可能是:当切向气体压力增加时,更多的冷却介质克服材料蒸汽反冲压力并进入到切缝内部进行有效冷却,减小了HAZ;同时造成激光能量损失大,材料去除量减少,所以槽深和深宽比逐渐减小。由表 4中的结果可计算出喷嘴角度、气体压力和喷嘴高度对HAZ的贡献大小分别为8.632%,40.012%和51.356%。因此,各因素对HAZ的影响大小排序为:喷嘴高度>气体压力>喷嘴角度。该结果与极差法所得结果一致,如图 4所示,结论可信。
对于深宽比,仅有喷嘴角度具有显著影响。喷嘴角度对槽深有一定影响,其余因素对槽深没有显著影响。从图 4可以看出,当喷嘴角度增加时HAZ逐渐增加,而槽深和深宽比先减小后迅速增大。原因可能是:当喷嘴角度较小时,CFRP壁面的液膜均匀性被破坏,更多的小液滴撞击CFRP表面后反弹到空气中,干扰激光传输,造成较大的激光能量损失,具体原因将在第2.2.1节中解释。切缝内部吸收的激光能量引起的热效应和水雾冷却效应达到一种相对的冷热平衡状态,有利于降低HAZ。CFRP吸收的激光能量减少,材料去除量减少;同时水雾射流运行距离长,导致机械应力冲击降低,所以槽深和深宽比有下降的趋势。
当喷嘴角度较大时,液膜稳定且小液滴对激光造成的损失减小,CFRP吸收的激光能量增加,提高了材料去除量;同时,水雾射流传播距离较短,水雾射流速度和冲击力大,可以通过机械应力冲蚀的方式提高材料去除率,所以提高了深宽比和槽深。但水雾将会在切缝中形成积水导致激光散射[17],破坏了切缝内部水雾冷却效应与激光热效应的冷热平衡状态,导致HAZ增加。由表 4中的结果可计算出喷嘴角度、气体压力和喷嘴高度对槽深的贡献大小分别为42.599%、33.547%和23.854%。因此,各因素对深度的影响大小排序为:喷嘴角度>气体压力>喷嘴高度。由表 4中的结果可计算出喷嘴角度、气体压力和喷嘴高度对深宽比的贡献大小分别为64.915%、15.374%和19.711%。因此,各因素对深宽比的影响大小排序为:喷嘴角度>喷嘴高度>气体压力。该结果与极差法所得结果一致,如图 4所示,结果可信。
众所周知,深宽比越大,在一定程度上代表加工质量越好。因此,本文中将进一步研究深宽比的最优水平组合。根据图 4c可知,当喷嘴角度50°、气体压力0.2 MPa和喷嘴高度为30 mm时可以获得更大的深宽比。16组正交实验中深宽比最大的参数组合为喷嘴角度50°、气体压力0.3 MPa和喷嘴高度为30 mm,此时深宽比为4.41。正交优化所得工艺参数组合深宽比为5.303,提高了20.958%。同时本文中对比了相同工艺参数下气体辅助激光加工的实验结果(HAZ为101.333 μm、深宽比为5.579、槽深为781.000 μm),如图 5所示。与气体辅助激光加工相比,水雾辅助激光加工CFRP的HAZ为85.875 μm,降低了15.255%;槽深为812.750 μm,提高了4.065%;但深宽比有所下降,降低了4.947%。水雾辅助激光加工产生的热损伤小主要是在于水雾对加工残余热量的及时冷却;水雾辅助激光加工CFRP的深宽比小、槽深大,这主要是因为水雾辅助时形成的槽宽大,导致深宽比下降。研究表明[22],较大的热损伤将会影响CFRP的机械强度,所以水雾更适合用来辅助激光加工CFRP。
2.2 水雾参数对激光传输的影响规律研究
当激光从空气介质中传输到水介质时,由于空气和水的光学特性不同,激光在空气-水界面时传输特性将会发生改变,如图 3b所示。此时激光传输将会出现3种情况:(a)部分激光被反射;(b)部分激光被水吸收;(c)大部分激光将会在空气-水界面发生折射。水对激光的反射和吸收都将造成激光能量损失,降低加工效率。而水对激光的折射将会改变激光焦点,甚至改变激光能量在空间的分布形态。本文中使用水雾辅助激光加工CFRP时,水雾射流在CFRP表面撞击将会形成流动水膜[19]。由于空气和水介质光学特性不同,将会对激光造成较大的影响。然而,现有的报道缺乏水雾对激光的影响规律的研究,这不利于加速推动水雾辅助激光加工的工业应用。因此,本节中将采用光束分析仪和激光功率计研究水雾对激光的影响规律。
为了避免高功率激光对检测设备造成损坏,将在低功率激光模式下进行测量, 并在光束分析仪的上方将会放置一激光能量衰减片(厚度为1 mm),同时光束分析仪位于激光焦点的下方。因此,本文中测得的激光光斑直径将会比焦点光斑直径大,但这并不影响总体的变化趋势。当无水雾辅助激光加工时,激光在空间的分布呈现高斯形态(后文简称为原始光斑直径),如图 6所示。图中纵坐标为相对数值,其值越接近1, 代表激光强度越高。同时采用激光功率计测量了不同条件下水雾对激光能量损失的影响。为了避免水雾影响设备的正常使用,需要在设备上方放置一石英玻璃,石英玻璃的透过率为93.967%。
2.2.1 喷嘴角度对激光的影响规律
图 7为喷嘴高度为30 mm、气体压力为0.2 MPa时不同喷嘴角度对激光分布形态的影响。从图 7可以看出,当喷嘴角度为20°时光斑直径最大,且大于激光在空气中的光斑直径。随着喷嘴角度的增加,光斑直径有变小的趋势,甚至比原始光斑直径更小。图 8a为激光经水雾损失后辐照在CFRP表面的激光功率。结合表 3,由图 8b可知,随着喷嘴角度增加, 水雾对激光功率的损失逐渐减小。原因可能是:当喷嘴角度较小时,根据勾股定理可知,为了保证偏置距离保持不变,势必要延长水雾射流在空气中传输距离,假设液滴在空间中充分发展,将会导致较多的小液滴在气体的作用下在空气中随机运动。当激光遇到液滴时传输方向会发生改变,同时大液滴撞击CFRP表面将会发生反弹和径向扩展[23],导致液膜内部的剪切力增加,CFRP表面形成液膜的均匀性将会被破坏,以上将会导致激光发散,扩大激光光斑直径。由于水对激光的吸收和小液滴对激光的发散将会造成能量损失严重,如图 8b所示。
当喷嘴角度较大时,液滴在水雾射流冲击处主要发生吸附和破碎,仅有较少部分破碎的小液滴才会被反弹到空气中,对激光的影响将会变小。结合表 3,由图 8b可知,当喷嘴角度为20°、30°、40°、50°时水雾对激光的损失率分别为4.563%、2.591%、1.782%和1.473%。因此,当喷嘴角度增加时,激光光斑直径和激光损失率均会减小。由于水的折射率(1.333)大于空气(1.000),当激光穿过空气-水膜界面时将会发生折射,缩小激光的光斑直径。因此,当喷嘴角度增加时光斑直径变小甚至小于原始光斑直径,如图 7所示。
2.2.2 喷嘴高度对激光的影响规律
图 9为喷嘴角度为50°、气体压力0.2 MPa时不同喷嘴高度(10 mm, 20 mm, 30 mm, 40 mm)对激光空间分布形态的影响。从图 9可知,当喷嘴高度为10 mm时,激光光斑直径最大且大于原始光斑直径。随着喷嘴高度增加,激光光斑直径逐渐减小且略小于原始光斑直径。图 8a表明,喷嘴高度变化时激光功率基本维持在一个水平,所以喷嘴高度对激光功率的影响较小。本文中将水雾射流撞击CFRP表面形成的3个区域分为:水雾射流冲击区、水膜流动区和自由区,如图 3a所示。水膜流动区的水层厚度薄、流速较快且稳定,对激光的影响小,适合用来辅助激光加工[19]。
研究表明,当喷嘴高度降低时壁面有效冷却范围将会降低[23]。随着喷嘴高度降低,稳定水膜流动区域范围将会减小。因此,当喷嘴角高为10 mm时,激光将会作用在自由区。自由区是指水雾射流冲击区和水膜流动区之外的区域。从图 3a可知,自由区由快速流动的细长水丝组成,该区域的液滴将会对激光传输造成严重的影响,使其激光随机传输,导致光斑直径增大。随着喷嘴高度增加,水膜流动区将会扩张,此后激光作用在水层厚度薄且快速流动的水膜流动区,在水的作用下激光光斑直径将会被缩小。因此,随着喷嘴高度增加,激光光斑直径逐渐减小。由第2.2.1节中的分析可知,当喷嘴角度较大时液滴在CFRP表面主要发生吸附和破碎作用,仅有少量的小液滴被反弹到空气中,此时激光功率的损失主要来自水膜对激光的反射和水对激光的吸收。因此,改变喷嘴高度时水雾对激光功率的影响保持在一个较为稳定的水平。
2.2.3 气体压力对激光的影响规律
图 10为喷嘴角度50°、喷嘴高度为30 mm时不同气体压力对激光在空间分布形态的影响。当气体压力增加时,激光光斑直径将会逐渐增加,且大于原始光斑直径,激光在空间的分布形态也更接近原始光斑形态,如图 10所示。随着气体压力的增加,激光损失率将会逐渐增加, 这是因为气体压力增加可以使较大的液滴破碎成更小的液滴,将会使喷雾中的液滴颗粒变得更加均匀[23]。由于气体的扩散性强,当气体压力增加时水雾射流直径将会被扩张;在气体的冲击作用下,部分较小的液滴更容易脱离水雾射流散入空气中。与喷嘴角度较小时情况类似,部分小液滴撞击CFRP将会发生反弹散入到空气中。当激光传输遇到液滴时将会发生折射、反射等光学现象,导致激光原有传输路径被改变,增加光斑直径。气压越大,飘入到空气中的小液滴越多。因此,随着气体压力的增加,激光光斑直径逐渐增加。当水雾射流撞击在CFRP表面时,液膜厚度会随着气体压力的增加而变薄[23],将会减小水膜对激光功率的影响,激光功率损失少。但随着气体压力增大,飘入到空气中的液滴增多,将会增加液滴对激光的散射严重,激光功率损失增加。因此,当气体压力为0.2 MPa, 0.3 MPa, 0.4 MPa和0.5 MPa时,激光损失率分别为1.473%, 1.649%, 1.899%和1.983%,如图 8b所示。
2.3 水雾辅助激光加工CFRP多元线性回归分析
为了评估激光加工CFRP的加工质量,需要对CFRP横截面打磨、测量,这将会增加一系列繁琐的操作且耗时,还会增加加工成本。为了减少加工后处理成本,本文中采用多元线性回归方法建立了喷嘴高度h、喷嘴角度ϕ和气体压力p与HAZ和槽深之间的经验公式。深宽比可以通过预测的槽深和槽宽计算,因此,本文中将不讨论深宽比的经验公式。正交实验所得HAZ和槽深的数据如表 5所示。假设CFRP的HAZ宽度H和槽深G的经验公式分别为[24-25]:
H=β1hβ2ϕβ3pβ4 (1) G=γ1hγ2ϕγ3pγ4 (2) 表 5 水雾辅助激光加工CFRP正交实验的实验数据表Table 5. Orthogonal experimental data sheet of water-mist-assisted laser processing of CFRPgroup number nozzle angle/(°) gas pressure/MPa nozzle height/mm cross section HAZ/μm groove depth/μm 1 20 0.2 10 98.625 745.000 2 20 0.3 20 95.375 592.750 3 20 0.4 30 102.875 611.250 4 20 0.5 40 101.125 548.750 5 30 0.2 20 106.125 613.750 6 30 0.3 10 98.625 676.750 7 30 0.4 40 101.625 555.250 8 30 0.5 30 96.125 633.750 9 40 0.2 30 102.625 681.000 10 40 0.3 40 103.875 619.750 11 40 0.4 10 93.625 666.250 12 40 0.5 20 99.875 632.000 13 50 0.2 40 112.250 775.250 14 50 0.3 30 105.375 771.750 15 50 0.4 20 97.625 655.750 16 50 0.5 10 95.500 666.000 式中,β1,β2,β3,β4,γ1,γ2,γ3,γ4为待求解常数系数。
将(1)式、(2)式两边同时取以10为底的对数,由对数运算法则可得:
lgH=lgβ1+β2lgh+β3lgϕ+β4lgp (3) lgG=lgγ1+γ2lgh+γ3lgϕ+γ4lgp (4) 为了便于求解,令Y1=lgH,K1=lgβ1,X1=lgh, X2=lgϕ,X3=lgp,Y2=lgG,K2=lgγ1上述(3)式、(4)式可改写为:
Y1=K1+β2X1+β3X2+β4X3 (5) Y2=K2+γ2X1+γ3X2+γ4X3 (6) 求解之前需将表 5中的数据转换为以10为底的对数关系数据,并代入到(5)式和(6)式,求解线性方程组,可得到以下关系式:
Y1=1.854+0.055X1+0.026X2−0.073X3,(R2=0.729) (7) Y2=2.598−0.055X1+0.144X2−0.147X3,(R2=0.590) (8) 最后将(7)式、(8)式代入到(3)式、(4)式中,并求解出HAZ宽度H和槽深G的经验公式分别为:
H=101.854h0.055ϕ0.026p−0.073,(R2=0.729) (9) G=102.598h−0.055ϕ0.144p−0.147,(R2=0.590) (10) 相关性检验,查表可知:F0.01(3, 12)=5.950, F0.05(3, 12)=3.490;FH=10.747>F0.01(3, 12)=5.950,所以经验公式(9)式具有高度显著;F0.01(3, 12)=5.950>FG=5.763>F0.05(3, 12)=3.490,所以经验公式(10)式是显著的。
本文中通过多元线性回归方法建立了CFRP的HAZ和槽深的经验公式,从相关性检验可以看出,所建立的经验公式中,HAZ的经验公式具有高度显著;槽深的经验公式相关性为显著,比HAZ的相关性低;根据方程拟合的R2可以看出,HAZ的经验公式R2较大,拟合精度较高,参考价值较高;槽深的R2小于HAZ的R2,说明HAZ的经验公式精度要高于槽深的经验公式精度。这些经验公式可以在未对CFRP做后处理便推算出切缝内部特征,尤其是对力学性能影响较大的HAZ。预测的结果可以减少后处理程序,节约加工成本;同时可以对加工参数的设置提供参考。
3. 结论
(a) 通过光束分析仪研究了水雾参数对激光光斑直径的影响。随着喷嘴高度、喷嘴角度增加,激光光斑直径不断减小;随着气体压力增加,激光光斑直径不断增加。
(b) 通过激光功率计研究了水雾参数对激光功率的影响。随着喷嘴高度增加,激光损失率变化较小;随着喷嘴角度增加和气体压力减小,激光损失率逐渐减小。
(c) 通过正交实验分析了水雾参数对加工质量的影响规律和主效应,喷嘴角度对深宽比具有显著性影响。当喷嘴角度50°、气体压力0.2 MPa和喷嘴高度为30 mm时, 可以获得最大5.303的深宽比,此时激光损失率为1.473%。与气体辅助激光加工CFRP相比,水雾辅助激光加工可以获得更小的HAZ和更大的槽深。
(d) 通过多元非线性回归分析建立了工艺参数(喷嘴高度、喷嘴角度和气体压力)和加工质量(HAZ和槽深)之间的经验公式,为预测水雾辅助激光加工CFRP的切缝内部特征提供了一种手段。
-
图 3 离焦量与能量密度引起的微观组织的变化[36]
a—离焦值为2mm,能量密度为33.74J/mm3 b—离焦值为2mm,能量密度为50.62J/mm3 c—离焦值为4mm,能量密度为50.62J/mm3
图 4 SLM成形Ti-6Al-4V原始态和热等静压处理后的拉伸应力-应变曲线对比[38]
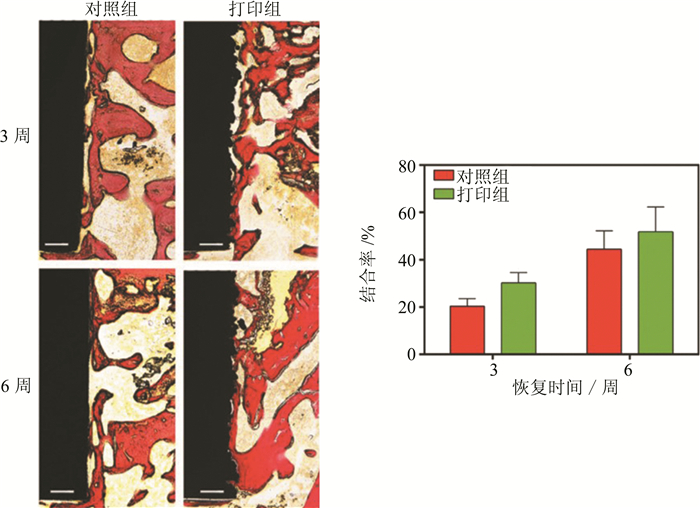
图 6 体内实验对照组与SLM打印组骨整合情况[86]
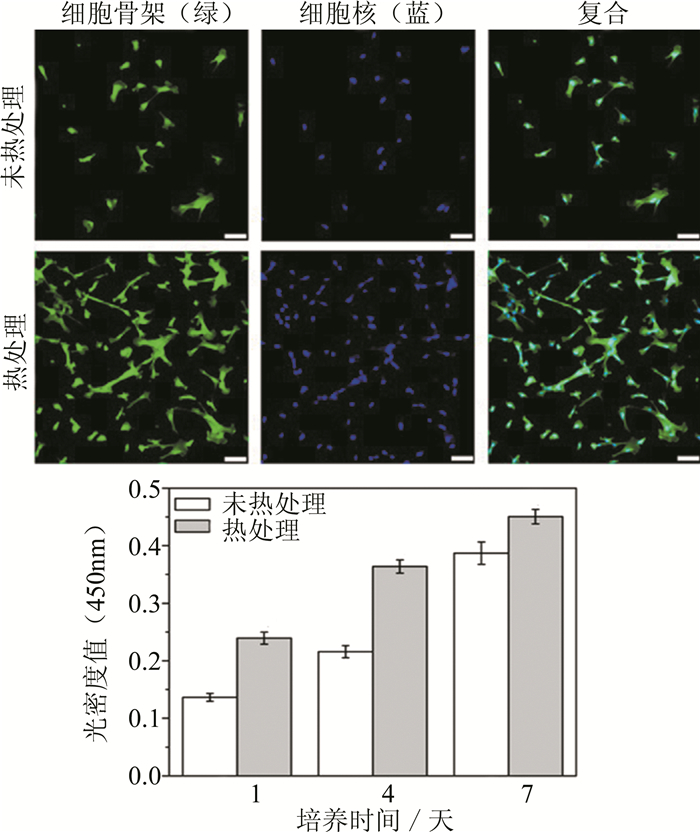
图 7 热处理与未热处理体外表面细胞生长对比情况[87]
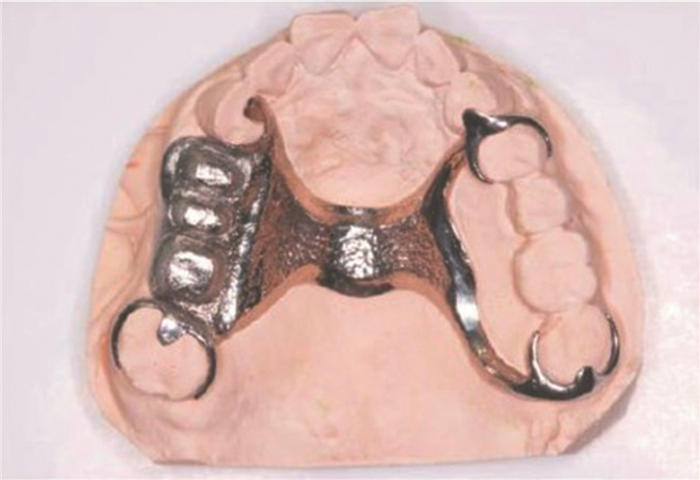
图 8 基于SLM成形的可摘除义齿[89]
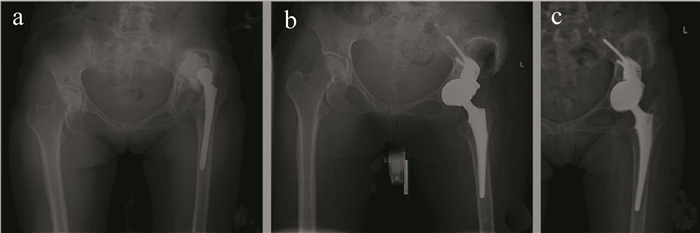
图 9 通过加强块植入重建髋关节案例[90]
a—术前CT关节图像b—术后随访CT关节图像c—末次随访CT关节图像
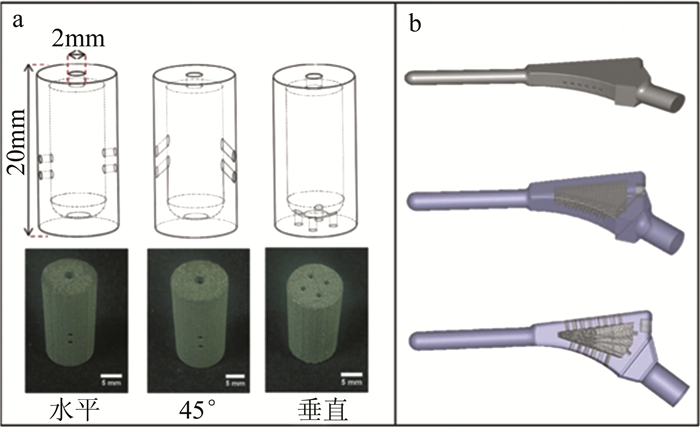
图 10 3种基于SLM成形多孔通道的载药结构
a—局部载药结构设计b—整体结构模型[92]
表 1 工艺参量对Ti-6Al-4V成形密度的影响研究
作者/时间 主要考察参量 主要结果/结论 THIJS[3] /2010 扫描策略 对比单向、z形及z形具有一定旋转角的3种不同扫描策略, z形旋转扫描具有更高的致密度,可达99.9% BARTOLOMEU[4]/2016 功率、间距、速度 利用数理统计方法分析了各参量之间存在相互作用,相同能量密度下,速度比间距对密度的影响更大 SHI[5]/ 2016
SHI[6]/2018层厚 高功率(400W)、高层厚(200μm)、搭配大光斑直径, 分别实现了粗粉(53μm~106μm)99.95%和99.99%相对密度的成形 HAN[7]/2017 速度、间距 搭配不同参量,能量密度在120J/mm3~190J/mm3之间为Ti-6Al-4V最佳工艺窗口范围,相对密度大于99.95% 表 2 常见不同单元结构类型尺寸设计的相关制造性能参考值[47]
单元类型 孔径/μm 结构大小/μm 多孔度/% 杨氏模量/
GPa屈服强度/
MPa参考文献 设计值 测量值 设计值 测量值 设计值 测量值 立方体 348~720 451~823 1452~1080 1413~1020 65~90 63~87 1.76~4.62 29~110 [48-49] 550, 800 — 300, 400 — 70.3~70.7 70.2~68.7 5.1~6.7 155~164 [50] 1000~2040 765~1020 450, 800 466~941 60.91~75.83 49.75~59.32 0.57~2.92 7.28~163.02 [51] 菱形金刚石 277~600 240~564 923~600 958~641 89~63 89~64 0.39~3.3 7~70 [48] — 670~1820 — 420~540 — 87~60 0.4~6.5 11.4~99.7 [52] 截头立方体 1720~1370 1625~1426 180~530 331~620 94~76 91~80 0.99~3.19 10~40 [48] 截头立方八面体 876~807 862~1049 324~564 862~1049 82~64 81~64 2.37~4.62 25~100 [48] 菱形十二面体 1250~950 1299~1058 250~550 246~506 90~66 89~68 0.22~2.97 7~88 [48] — — — 67~129 — 84~67 0.55 — [53] 小斜方截半立方体 820~670 877~794 380~530 348~438 84~64 89~68 2.23~4.4 39~93 [48] 十二面体 — 150 — 500 — 80 1.22 12.7 [54] 500, 450 560, 486 120, 170 140, 216 — 68~84 0.55~3.49 15.8~91.8 [55] 四面体 500 — 0.2~0.39 — 50~75 — 4.3~1.9 57~156 [56] 八角框架 770 — 0.2~0.4 — 50~75 — 4.6~1.2 34~172 [56] 螺旋杆架 — — 0.9~1.1 — 55~60 55~61 3.4~26.3 103~402 [55] 三周期极小
曲面螺旋体— 560~1600 — — — 80~95 0.13~1.25 6.5~81.3 [57] 三周期极小
曲面菱体— 480~1450 — — — 80~95 0.12~1.25 4.66~69.21 [57] 表 3 不同后处理方式对疲劳特性影响[71]
处理方式 效果 原因 退火 不明显 应力得到消除,但硬度下降 电解质抛光 不明显 暴露了内部存在的缺陷 喷丸 提高 引入了表面压缩应力 热等静压 提高 消除了内部孔隙 表 4 SLM成形Ti-6Al-4V的相关耐腐蚀性行为对比研究
作者/时间 腐蚀介质 主要工作 主要结论 YE[78]/2014 0.9%NaCl 对比了SLM热处理前后与铸造件的电化学腐蚀行为 SLM强于铸造,热处理后重新排布了内部组织,消除了内部应力,耐腐蚀能力得到提高 DAI [79]/2016 3.5%NaCl 对比了SLM与商业5级样的电化学腐蚀行为 SLM耐腐蚀性差,α′相的存在以及β相组织过少, 降低了耐腐蚀性能 CHEN[80]/2016 模拟体液 对比了SLM成形Ti-6Al-4V异向面与商业轧制件的电化学腐蚀行为 耐腐蚀性表现出方向异性,但整体耐腐蚀性仍强于商业轧制件 ZHAO[81]/2017 模拟体液 对比了SLM、EBM、锻造方式下Ti-6Al-4V的腐蚀行为 EBM腐蚀速率与电位有关,SLM耐腐蚀总是强于铸造,三者腐蚀速率都远低于美国腐蚀工程师协会标准 LONGHITANO[82]/2018 磷酸缓冲盐溶液 对比了不同热处理手段对于SLM成形Ti-6Al-4V的电化学腐蚀行为 热处理后的β相组织降低了耐腐蚀性能,提出一种阳极氧化的表面处理手段,处理后耐腐蚀能力与常规锻造件相当 -
[1] GEETHA M, SINGH A K, ASOKAMANI R, et al. Ti based biomaterials, the ultimate choice for orthopaedic implants-A review[J]. Progress in Materials Science, 2009, 54(3): 397-425. DOI: 10.1016/j.pmatsci.2008.06.004
[2] ZHOU B, ZHOU J, LI H, et al. A study of the microstructures and mechanical properties of Ti6Al4V fabricated by SLM under vacuum[J]. Materials Science and Engineering, 2018, A724: 1-10. http://cn.bing.com/academic/profile?id=3dc784618d0bbe79890d242723991b9f&encoded=0&v=paper_preview&mkt=zh-cn
[3] THIJS L, VERHAEGHE F, CRAEGHS T, et al. A study of the microstructural evolution during selective laser melting of Ti-6Al-4V[J]. Acta Materialia, 2010, 58(9): 3303-3312. DOI: 10.1016/j.actamat.2010.02.004
[4] BARTOLOMEU F, FARIA S, CARVALHO O, et al. Predictive models for physical and mechanical properties of Ti6Al4V produced by selective laser melting[J]. Materials Science and Engineering, 2016, A663: 181-192. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=a08fc51e5f51477f199a62f5b6355ca9
[5] SHI X, MA S, LIU C, et al. Performance of high layer thickness in selective laser melting of Ti-6Al-4V[J]. Materials (Basel, Switzerland), 2016, 9(12): 975-990. DOI: 10.3390/ma9120975
[6] SHI W, LIU Y, SHI X, et al. Beam diameter dependence of performance in thick-layer and high-power selective laser melting of Ti-6Al-4V[J]. Materials, 2018, 11(7): 1237-1254. DOI: 10.3390/ma11071237
[7] HAN J, YANG J J, YU H, et al. Microstructure and mechanical property of selective laser melted Ti6Al4V dependence on laser energy density[J]. Rapid Prototyping Journal, 2017, 23(2): 217-226. DOI: 10.1108/RPJ-12-2015-0193
[8] KASPEROVICH G, HAUSMANN J. Improvement of fatigue resistance and ductility of TiAl6V4 processed by selective laser melting[J]. Journal of Materials Processing Technology, 2015, 220: 202-214. DOI: 10.1016/j.jmatprotec.2015.01.025
[9] ZHANG Q, LIANG Z, CAO M, et al. Microstructure and mechanical properties of Ti6Al4V alloy prepared by selective laser melting combined with precision forging[J]. Transactions of Nonferrous Metals Society of China, 2017, 27(5): 1036-1042. DOI: 10.1016/S1003-6326(17)60121-3
[10] THÖNE M, LEUDERS S, RIEMER A, et al. Influence of heat-treatment on selective laser melting products-e.g.Ti-6Al-4V[C]//Proceedings of the 23rd Annual International Solid Freeform Fabrication Symposium : An Additive Manufacturing Conference. Austin, USA: University of Texas, 2012: 492-496.
[11] HUSSEIN A, HAO L, YAN C, et al. Advanced lattice support structures for metal additive manufacturing[J]. Journal of Materials Processing Technology, 2013, 213(7): 1019-1026. DOI: 10.1016/j.jmatprotec.2013.01.020
[12] VRANCKEN B, BULS S, KRUTH J P. Preheating of selective laser melted Ti-6Al-4V: Microstructure and mechanical properties[C]// Proceedings of the 13th World Conference on Titanium. San Diego, USA: The Minerals, Metals & Materials Society, 2016: 1269-1277.
[13] LIU Y, YANG Y, WANG D. A study on the residual stress during selective laser melting (SLM) of metallic powder[J]. The International Journal of Advanced Manufacturing Technology, 2016, 87(1): 647-656. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=b10de11dcc20a2808a75c556b427ecbb
[14] CHENG B, SHRESTHA S, CHOU K. Stress and deformation evaluations of scanning strategy effect in selective laser melting[J]. Additive Manufacturing, 2016, 12: 240-251. DOI: 10.1016/j.addma.2016.05.007
[15] MISHUROVA T, CABEZA S, ARTZT K, et al. An assessment of subsurface residual stress analysis in SLM Ti-6Al-4V[J]. Materials, 2017, 10(4): 348-362. DOI: 10.3390/ma10040348
[16] PARRY L A, ASHCROFT I A, WILDMAN R D. Geometrical effects on residual stress in selective laser melting[J]. Additive Manufacturing, 2019, 25: 166-175. DOI: 10.1016/j.addma.2018.09.026
[17] SUN D, GU D, LIN K, et al. Selective laser melting of titanium parts: Influence of laser process parameters on macro- and microstructures and tensile property[J]. Powder Technology, 2019, 342: 371-379. DOI: 10.1016/j.powtec.2018.09.090
[18] LIU Y J, LI X P, ZHANG L C, et al. Processing and properties of topologically optimised biomedical Ti-24Nb-4Zr-8Sn scaffolds manufactured by selective laser melting[J]. Materials Science and Engineering, 2015, A642: 268-278. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=d7470c1f0d2580c7160f0d86ce2accd0
[19] WANG X L. Study on process optimization and property of titanium alloy manufactured by selective laser melting[D].Guangzhou: South China University of Technology, 2016: 60-65(in Chinese).
[20] van BAEL S, KERCKHOFS G, MOESEN M, et al. Micro-CT-based improvement of geometrical and mechanical controllability of selective laser melted Ti6Al4V porous structures[J]. Materials Science and Engineering, 2011, A528(24): 7423-7431. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=1b432a991132165cc36bf556c22e016a
[21] CHAN K S, KOIKE M, MASON R L, et al. Fatigue life of titanium alloys fabricated by additive layer manufacturing techniques for den-tal implants[J]. Metallurgical and Materials Transactions, 2013, A44(2): 1010-1022.
[22] DO D K, LI P. The effect of laser energy input on the microstructure, physical and mechanical properties of Ti-6Al-4V alloys by selective laser melting[J]. Virtual and Physical Prototyping, 2016, 11(1): 41-47. DOI: 10.1080/17452759.2016.1142215
[23] WYCISK E, EMMELMANN C, SIDDIQUE S, et al. High cycle fatigue (HCF) performance of Ti-6Al-4V alloy processed by selective laser melting[J]. Advanced Materials Research, 2013, 816/817: 134-139. DOI: 10.4028/www.scientific.net/AMR.816-817.134
[24] CHEN C, HAO Y, BAI X, et al. 3-D printed porous Ti6Al4V cage: Effects of additive angle on surface properties and biocompatibility; bone ingrowth in Beagle tibia model[J]. Materials & Design, 2019, 175: 107824. https://www.researchgate.net/publication/332796686_3D_printed_porous_Ti6Al4V_cage_Effects_of_additive_angle_on_surface_properties_and_biocompatibility_bone_ingrowth_in_Beagle_tibia_model
[25] WANG Z, XIAO Z, TSE Y, et al. Optimization of processing parameters and establishment of a relationship between microstructure and mechanical properties of SLM titanium alloy[J]. Optics & Laser Technology, 2019, 112: 159-167. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=84ad8b49ba2baf58a4c90a43f0cef28c
[26] GE Y N, WU M P, MAO Y Y, et al. Effect of scanning strategy on forming precision of titanium alloy by selective laser melting [J]. Laser & Optoelectronics Progress, 2018, 55(9): 091403(in Chin-ese). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=jgygdzxjz201809032
[27] YASA E, DECKERS J. The investigation of the influence of laser remelting on density, surface quality and microstructure of selective laser melting parts[J]. Rapid Prototyping Journal, 2011, 17(5): 312-327. DOI: 10.1108/13552541111156450
[28] LIU J H, ZHU H H, HU Zh H, et al. Control of elevated edge in selective laser melt molding[J]. Chinese Journal of Lasers, 2017, 44(12): 1202007(in Chinese). DOI: 10.3788/CJL201744.1202007
[29] DECKERS J, YASA E, KRUTH J P. The investigation of the influence of laser re-melting on density, surface quality and microstructure of selective laser melting parts[J]. Rapid Prototyping Journal, 2011, 17(5): 312-327. DOI: 10.1108/13552541111156450
[30] VAITHILINGAM J, GOODRIDGE R D, HAGUE R J M, et al. The effect of laser remelting on the surface chemistry of Ti6al4V components fabricated by selective laser melting[J]. Journal of Materials Processing Technology, 2016, 232: 1-8. DOI: 10.1016/j.jmatprotec.2016.01.022
[31] VILARO T, COLIN C, BARTOUT J D. As-fabricated and heat-treated microstructures of the Ti-6Al-4V alloy processed by selective laser melting[J]. Metallurgical and Materials Transactions, 2011, A42(10): 3190-3199. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=6e182ca48b39c719369f5254062d3a24
[32] KHORASANI A, GIBSON I, GOLDBERG M, et al. On the role of different annealing heat treatments on mechanical properties and microstructure of selective laser melted and conventional wrought Ti-6Al-4V[J]. Rapid Prototyping Journal, 2017, 23(2): 295-304. DOI: 10.1108/RPJ-02-2016-0022
[33] CAIN V, THIJS L, VAN HUMBEECK J, et al. Crack propagation and fracture toughness of Ti6Al4V alloy produced by selective laser melting[J]. Additive Manufacturing, 2015, 5: 68-76. DOI: 10.1016/j.addma.2014.12.006
[34] SHUNMUGAVEL M, POLISHETTY A, LITTLEFAIR G. Microstructure and mechanical properties of wrought and additive manufactured Ti-6Al-4V cylindrical bars[J]. Procedia Technology, 2015, 20: 231-236. DOI: 10.1016/j.protcy.2015.07.037
[35] SIMONELLI M, TSE Y Y, TUCK C. Effect of the build orientation on the mechanical properties and fracture modes of SLM Ti-6Al-4V[J]. Materials Science and Engineering, 2014, A616: 1-11. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=8a1dd1658903325fd0e28ea615e85090
[36] XU W, SUN S, ELAMBASSERIL J, et al. Ti-6Al-4V additively manufactured by selective laser melting with superior mechanical properties[J]. JOM, 2015, 67(3): 668-673. DOI: 10.1007/s11837-015-1297-8
[37] ALI H, MA L, GHADBEIGI H, et al. In-situ residual stress reduction, martensitic decomposition and mechanical properties enhancement through high temperature powder bed pre-heating of selective laser melted Ti-6Al-4V[J]. Materials Science and Engineering, 2017, A695: 211-220. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cc06ddaf7e6c6c0442204bbfe3d82c56
[38] QIU C, ADKINS N J E, ATTALLAH M M. Microstructure and tensile properties of selectively laser-melted and of HIPed laser-melted Ti-6Al-4V[J]. Materials Science and Engineering, 2013, A578: 230-239. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=5fdff42bf2c3adee4007e299f2204bff
[39] LI W X, YI D Q, LIU H Q, et al. Effect of the heat treatment process on microstructure and mechanical properties of the TC4 alloy processed by selective laser melting [J]. Materials Science and Engineering of Powder Metallurgy, 2017, 22(1): 70-78(in Chinse). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=fmyjclkxygc201701011
[40] ROOHANI-ESFAHANI S, NEWMAN P, ZREIQAT H. Design and fabrication of 3-D printed scaffolds with a mechanical strength comparable to cortical bone to repair large bone defects[J]. Scientific Reports, 2016, 6: 19468. DOI: 10.1038/srep19468
[41] SHIBATA Y, TANIMOTO Y, MARUYAMA N, et al. A review of improved fixation methods for dental implants. Part Ⅱ: Biomechanical integrity at bone-implant interface[J]. Journal of Prosthodontic Research, 2015, 59(2): 84-95. DOI: 10.1016/j.jpor.2015.01.003
[42] KELLY A, HIDEO N. Metallic scaffolds for bone regeneration[J]. Materials, 2009, 2(3):790-832. DOI: 10.3390/ma2030790
[43] MARIN E, FUSI S, PRESSACCO M, et al. Characterization of ce-llular solids in Ti6Al4V for orthopaedic implant applications: Trabecular titanium[J].Journal of the Mechanical Behavior of Biome-dical Materials, 2010, 3(5): 373-381. DOI: 10.1016/j.jmbbm.2010.02.001
[44] BARTOLOMEU F, COSTA M M, GOMES J R, et al. Implant surface design for improved implant stability-A study on Ti6Al4V dense and cellular structures produced by selective laser melting[J]. Tribology International, 2019, 129: 272-282. DOI: 10.1016/j.triboint.2018.08.012
[45] WANG Y, SHEN Y, WANG Z, et al. Development of highly porous titanium scaffolds by selective laser melting[J]. Materials Letters, 2010, 64(6):674-676. DOI: 10.1016/j.matlet.2009.12.035
[46] SHENG Z, WEI Q, CHENG L, et al. Effects of scan line spacing on pore characteristics and mechanical properties of porous Ti6Al4V implants fabricated by selective laser melting[J]. Materials & Design, 2014, 63(21): 185-193. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=9fe1f2bbbe9abfcfd37eefe3b6fcee06
[47] ZHANG X Y, FANG G, ZHOU J. Additively manufactured scaffolds for bone tissue engineering and the prediction of their mechanical behavior: A review[J]. Materials, 2017, 10(1):50-52. http://cn.bing.com/academic/profile?id=b61a8960d939bb09a505db77265c8d1b&encoded=0&v=paper_preview&mkt=zh-cn
[48] AHMADI S, YAVARI S, WAUTHLE R, et al. Additively manufactured open-cell porous biomaterials made from six different space-filling unit cells: The mechanical and morphological properties[J]. Materials, 2015, 8(4):1871-1896. DOI: 10.3390/ma8041871
[49] KADKHODAPOUR J, MONTAZERIAN H, DARABI A C, et al. Failure mechanisms of additively manufactured porous biomaterials: Effects of porosity and type of unit cell[J]. Journal of the Mechanical Behavior of Biomedical Materials, 2015, 50: 180-191. DOI: 10.1016/j.jmbbm.2015.06.012
[50] WIEDING J, JONITZ A, BADER R. The effect of structural design on mechanical properties and cellular response of additive manufactured titanium scaffolds[J]. Materials, 2012, 5(8):1336-1347. DOI: 10.3390/ma5081336
[51] PARTHASARATHY J, STARLY B, RAMAN S, et al. Mechanical evaluation of porous titanium (Ti6Al4V) structures with electron beam melting (EBM)[J]. Journal of the Mechanical Behavior of Biomedical Materials, 2010, 3(3): 249-259. DOI: 10.1016/j.jmbbm.2009.10.006
[52] WEIβMANN V, BADER R, HANSMANN H, et al. Influence of the structural orientation on the mechanical properties of selective laser melted Ti6Al4V open-porous scaffolds[J]. Materials & Design, 2016, 95: 188-197. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=4fd010567929c92c0f85126fab540e66
[53] HEINL P, KÖRNER C, SINGER R F. Selective electron beam melting of cellular titanium: Mechanical properties[J]. Advanced Engineering Materials, 2008, 10(9):882-888. DOI: 10.1002/adem.200800137
[54] CAMPOLI G, BORLEFFS M S, AMIN YAVARI S, et al. Mechanical properties of open-cell metallic biomaterials manufactured using additive manufacturing[J]. Materials & Design, 2013, 49(C):957-965. http://cn.bing.com/academic/profile?id=640cbb9956a967e23fa29060dbaa81ee&encoded=0&v=paper_preview&mkt=zh-cn
[55] WAUTHLE R, JOHAN V D S, AMIN Y S, et al. Additively manufactured porous tantalum implants[J]. Acta Biomaterialia, 2015, 14:217-225. DOI: 10.1016/j.actbio.2014.12.003
[56] ARABNEJAD S, JOHNSTON R B, PURA J A, et al. High-strength porous biomaterials for bone replacement: A strategy to assess the interplay between cell morphology, mechanical properties, bone ingrowth and manufacturing constraints[J]. Acta Biomaterialia, 2015, 30(8):345-356. http://cn.bing.com/academic/profile?id=9127819148b348af8e5e7d4349c24e30&encoded=0&v=paper_preview&mkt=zh-cn
[57] YAN C, HAO L, HUSSEIN A, et al. Ti-6Al-4V triply periodic minimal surface structures for bone implants fabricated via selective laser melting[J]. Journal of the Mechanical Behavior of Biomedical Materials, 2015, 51:61-73. DOI: 10.1016/j.jmbbm.2015.06.024
[58] CHALLIS V J, XU X, LAI C Z, et al. High specific strength and stiffness structures produced using selective laser melting[J]. Materials & Design, 2014, 63(2): 783-788. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=281ed7cbbaf18627ebae78550625c09a
[59] FOUSOVÁ M, VOJTĚCH D, KUBÁSEK J, et al. Promising characteristics of gradient porosity Ti-6Al-4V alloy prepared by SLM process[J]. Journal of the Mechanical Behavior of Biomedical Materials, 2017, 69: 368-376. DOI: 10.1016/j.jmbbm.2017.01.043
[60] HAN Ch J, LI Y, WANG Q, et al. Continuous functionally graded porous titanium scaffolds manufactured by selective laser melting for bone implants[J]. Journal of the Mechanical Behavior of Biomedical Materials, 2018, 80: 119-127. DOI: 10.1016/j.jmbbm.2018.01.013
[61] BARTOLOMEU F, SAMPAIO M, CARVALHO O, et al. Tribological behavior of Ti6Al4V cellular structures produced by selective laser melting[J]. Journal of the Mechanical Behavior of Biomedical Materials, 2017, 69: 128-134. DOI: 10.1016/j.jmbbm.2017.01.004
[62] CHAN K S, KOIKE M, MASON R L, et al. Fatigue life of titanium alloys fabricated by additive layer manufacturing techniques for den-tal implants[J]. Metallurgical and Materials Transactions, 2013, A44(2): 1010-1022.
[63] EDWARDS P, RAMULU M. Fatigue performance evaluation of selective laser melted Ti-6Al-4V[J]. Materials Science and Engineering, 2014, A598: 327-337. http://cn.bing.com/academic/profile?id=e918a5fdc5ef28a0a6984edd0ad6c2ff&encoded=0&v=paper_preview&mkt=zh-cn
[64] van HOOREWEDER B, APERS Y, LIETAERT K, et al. Improving the fatigue performance of porous metallic biomaterials produced by selective laser melting[J]. Acta Biomaterialia, 2017, 47: 193-202. DOI: 10.1016/j.actbio.2016.10.005
[65] DALLAGO M, ZANINI F, CARMIGNATO S, et al. Effect of the geometrical defectiveness on the mechanical properties of SLM biomedical Ti6Al4V lattices[J]. Procedia Structural Integrity, 2018, 13: 161-167. DOI: 10.1016/j.prostr.2018.12.027
[66] YADROITSEV I, KRAKHMALEV P, YADROITSAVA I, et al. Qualification of Ti6Al4V ELI alloy produced by laser powder bed fusion for biomedical applications[J]. JOM, 2018, 70(3): 372-377. DOI: 10.1007/s11837-017-2655-5
[67] HACKEL L, RANKIN J R, RUBENCHIK A, et al. Laser peening: A tool for additive manufacturing post-processing[J]. Additive Manufacturing, 2018, 24: 67-75. DOI: 10.1016/j.addma.2018.09.013
[68] LEUDERS S, THÖNE M, RIEMER A, et al. On the mechanical behaviour of titanium alloy TiAl6V4 manufactured by selective laser melting: Fatigue resistance and crack growth performance[J]. International Journal of Fatigue, 2013, 48: 300-307. DOI: 10.1016/j.ijfatigue.2012.11.011
[69] ZHANG H, DONG D, SU S, et al. Experimental study of effect of post processing on fracture toughness and fatigue crack growth performance of selective laser melting Ti-6Al-4V [DB/OL].(2019-01-03)[2019-09-25].https://www.sciencedirect.com/science/article/pii/S1000936119300196.
[70] DALLAGO M, FONTANARI V, TORRESANI E, et al. Fatigue and biological properties of Ti-6Al-4V ELI cellular structures with variously arranged cubic cells made by selective laser melting[J]. Journal of the Mechanical Behavior of Biomedical Materials, 2018, 78: 381-394. DOI: 10.1016/j.jmbbm.2017.11.044
[71] BENEDETTI M, FONTANARI V, BANDINI M, et al. Low- and high-cycle fatigue resistance of Ti-6Al-4V ELI additively manufactured via selective laser melting: Mean stress and defect sensitivity[J]. International Journal of Fatigue, 2018, 107: 96-109. DOI: 10.1016/j.ijfatigue.2017.10.021
[72] GVNTHER J, KREWERTH D, LIPPMANN T, et al. Fatigue life of additively manufactured Ti-6Al-4V in the very high cycle fatigue regime[J]. International Journal of Fatigue, 2017, 94: 236-245. DOI: 10.1016/j.ijfatigue.2016.05.018
[73] NICOLETTO G, MAISANO S, ANTOLOTTI M, et al. Influence of post fabrication heat treatments on the fatigue behavior of Ti-6Al-4V produced by selective laser melting[J]. Procedia Structural Integrity, 2017, 7: 133-140. DOI: 10.1016/j.prostr.2017.11.070
[74] ZHAO D, HUANG Y, AO Y, et al. Effect of pore geometry on the fatigue properties and cell affinity of porous titanium scaffolds fabricated by selective laser melting[J]. Journal of the Mechanical Behavior of Biomedical Materials, 2018, 88: 478-487. DOI: 10.1016/j.jmbbm.2018.08.048
[75] AMIN YAVARI S, AHMADI S M, WAUTHLE R, et al. Relationship between unit cell type and porosity and the fatigue behavior of selective laser melted meta-biomaterials[J]. Journal of the Mechanical Behavior of Biomedical Materials, 2015, 43: 91-100. DOI: 10.1016/j.jmbbm.2014.12.015
[76] BARTOLOMEU F, BUCIUMEANU M, PINTO E, et al. Wear behavior of Ti6Al4V biomedical alloys processed by selective laser melting, hot pressing and conventional casting[J]. Transactions of Nonferrous Metals Society of China, 2017, 27(4): 829-838. DOI: 10.1016/S1003-6326(17)60060-8
[77] LU Y Q, MEI Z Y, ZHANG Q Q, et al. Friction and wear performance test of SLM formed titanium alloy and cast titanium alloy[C]//The 12th National Conference on Prosthodontics.Beijing: Chinese Stomatological Association, 2018: 117(in Chinese).
[78] YE Z H. The personalized design and process research of selective laser melting manufacturing of Ti-6Al-4V tibial implant[D].Guangzhou: South China University of Technology, 2014: 68-69(in Chinese).
[79] DAI N, ZHANG L, ZHANG J, et al. Corrosion behavior of selective laser melted Ti-6Al-4V alloy in NaCl solution[J]. Corrosion Science, 2016, 102: 484-489. DOI: 10.1016/j.corsci.2015.10.041
[80] CHEN L Y, HUANG J C, LIN C H, et al. Anisotropic response of Ti-6Al-4V alloy fabricated by 3-D printing selective laser melting[J]. Materials Science & Engineering, 2017, A682: 389-395. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=ff4d0971ce523eec3452cd4c6e5fe42b
[81] ZHAO B, WANG H, QIAO N, et al. Corrosion resistance characteristics of a Ti-6Al-4V alloy scaffold that is fabricated by electron beam melting and selective laser melting for implantation in vivo[J]. Materials Science and Engineering, 2017, C70: 832-841. http://cn.bing.com/academic/profile?id=f77c8c8bb91728fc2add21a00b1f04cf&encoded=0&v=paper_preview&mkt=zh-cn
[82] LONGHITANO G A, ARENAS M A, CONDE A, et al. Heat treatments effects on functionalization and corrosion behavior of Ti-6Al-4V ELI alloy made by additive manufacturing[J]. Journal of Alloys and Compounds, 2018, 765: 961-968. DOI: 10.1016/j.jallcom.2018.06.319
[83] TOPTAN F, ALVES A C, CARVALHO Ó, et al. Corrosion and tribocorrosion behaviour of Ti6Al4V produced by selective laser melting and hot pressing in comparison with the commercial alloy[J]. Journal of Materials Processing Technology, 2019, 266: 239-245. DOI: 10.1016/j.jmatprotec.2018.11.008
[84] VAITHILINGAM J, PRINA E, GOODRIDGE R D, et al. Surface chemistry of Ti6Al4V components fabricated using selective laser melting for biomedical applications[J]. Materials Science and Engineering, 2016, C67: 294-303. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=57b51cb8abdf944f3924a1d78058ddbc
[85] RUI M, ZHENG X, ZHANG Y Q, et al. Three-dimensional printing porous titanium alloy scaffold repairs radial bone defect in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(18): 2789-2793(in Chinese). http://d.old.wanfangdata.com.cn/Periodical/xdkf201918002
[86] ZHANG J, ZHOU W, WANG H, et al. 3D-printed surface promoting osteogenic differentiation and angiogenetic factor expression of BMSCs on Ti6Al4V implants and early osseointegration in vivo[J]. Journal of Materials Science & Technology, 2019, 35(2): 336-343. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=clkxjsxb-e201902011
[87] WANG M, WU Y, LU S, et al. Fabrication and characterization of selective laser melting printed Ti-6Al-4V alloys subjected to heat treatment for customized implants design[J]. Progress in Natural Science: Materials International, 2016, 26(6): 671-677. DOI: 10.1016/j.pnsc.2016.12.006
[88] NANJIXIONG. Application of domestic medical institutions in 3-D printing human implants [EB/OL].(2016-10-13)[2019-09-02].www.nanjixiong.com(in Chinese).
[89] LIU Y F, WANG W N, YU H, et al. Study on the fit of titanium alloy removable partial denture framework fabricated by selective laser melting[J]. Journal of Practical Stomatology, 2017, 33(3): 302-305(in Chinese). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=sykqyxzz201703004
[90] FU J, NI M, CHEN J Y, et al. Early clinical outcomes of individua-lized 3-D printed porous Ti6Al4V augment reconstruction in severe acetabular bone defect[J].Chinese Journal Bone and Joint Surgery, 2018, 11(6): 401-407(in Chinese).
[91] HASSANIN H, FINET L, COX S C, et al. Tailoring selective laser melting process for titanium drug-delivering implants with releasing micro-channels[J]. Additive Manufacturing, 2018, 20: 144-155. DOI: 10.1016/j.addma.2018.01.005
[92] COX S C, JAMSHIDI P, EISENSTEIN N M, et al. Adding functionality with additive manufacturing: Fabrication of titanium-based antibiotic eluting implants[J]. Materials Science and Engineering, 2016, C64: 407-415. http://cn.bing.com/academic/profile?id=a8fda5c19a7020dd24d79f774d1ef879&encoded=0&v=paper_preview&mkt=zh-cn
-
期刊类型引用(1)
1. 周嘉,周辽,欧阳励,焦辉,黄宇星,龙芋宏. 基于CBDC优化超声辅助水下激光切割单晶硅的工艺. 激光技术. 2024(06): 913-921 .  本站查看
本站查看
其他类型引用(0)




 下载:
下载: