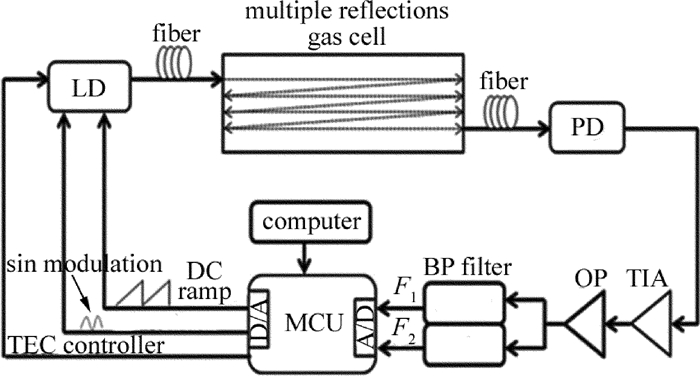
Research of propane detecting based on near-infrared laser spectral absorption
-
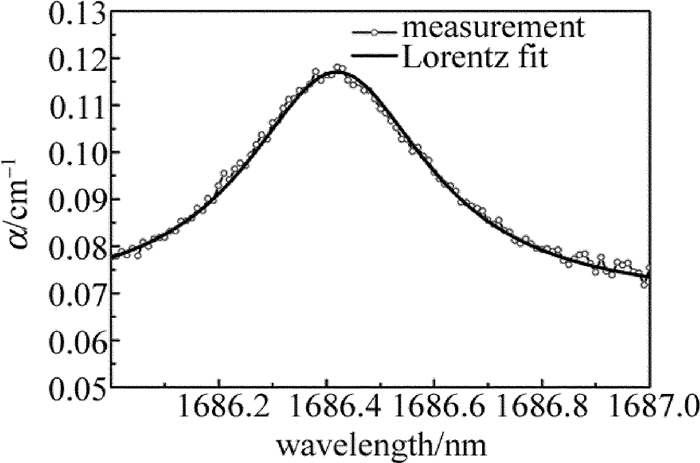
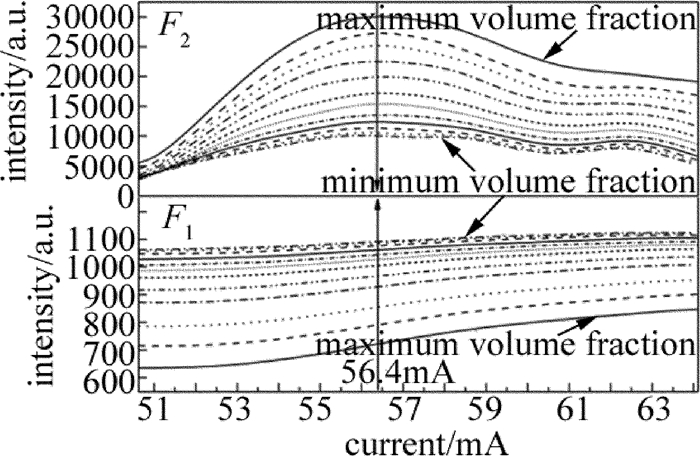
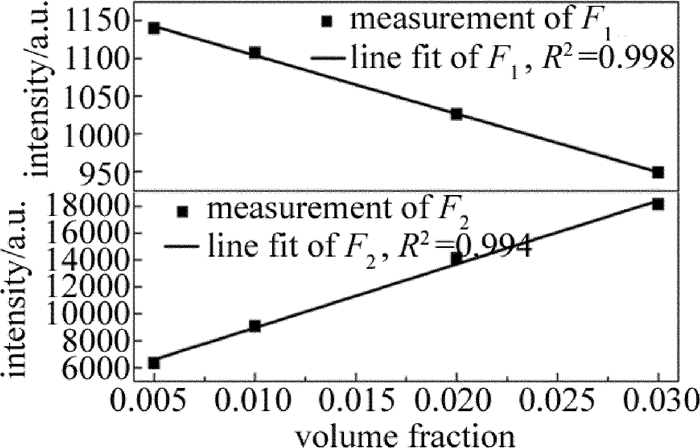
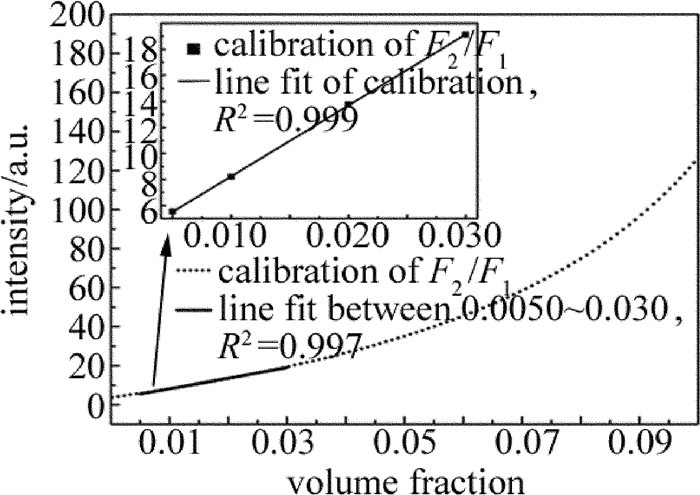
摘要: 为了对石油气挥发性有机化合物的主要成分进行实时监测,实现石油化工行业的安全生产,采用激光光谱分析技术、利用宽谱光源分析了丙烷在1686.00nm~1687.00nm波段的光谱吸收特征,获得了吸收系数随波长变化的洛伦兹线型,其半峰半宽为0.21nm。选择中心波长为1686.30nm的分布反馈式半导体激光器作为光源,在丙烷宽谱吸收峰范围内进行波长扫描,得到了一次谐波信号和二次谐波信号随丙烷体积分数的变化规律,并在丙烷的体积分数0.0050~0.0300范围内标定了二次谐波与一次谐波信号的比值与体积分数的线性关系。结果表明,实验系统有很好的稳定性与重复性,能够进行实时的丙烷在线检测。该研究为探测其它挥发性有机化合物气体提供了理论及实验参考。Abstract: In order to real-time detect the main component of volatile organic compounds of oil gas and realize production safety in petrochemical industry, laser spectrum analysis technique was applied. Firstly, the broadband optical source was utilized to analyze the spectral absorption features of propane between 1686.00nm and 1687.00nm. The relationship between absorption coefficient and wavelength was fit into Lorentz lineshape, with half width at half max of 0.21nm. A distributed feedback laser with center wavelength of 1686.30nm was chosen as the sigle mode light source, and laser wavelength scan was made within wide absorption spectrum of propane. The changing regularity of the first and second harmonic signal with volume fraction of propane was acquired. The linear relationship between the ratio of the second harmonic signal to the first harmonic signal and propane volume fraction was calibrated with propane volume fraction from 0.0050 to 0.0300. The results show that, the system has good stability and repeatability, and can be used to do propane online detection. The study can provide the reference of theory and experiment for detecting other volatile organic compound gases.
-
Keywords:
- spectroscopy /
- propane detection /
- tunable diode laser /
- near infrared /
- volatile organic compound
-
-
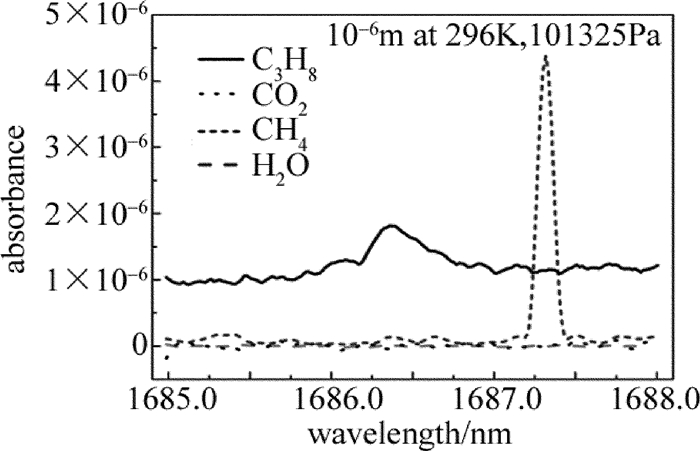
Figure 1. Absorption spectrum of propane, carbon dioxide, methane and water near 1686nm[16]
-
[1] DU Z H, ZHAI Y Q, LI J Y, et al. Techniques of on-line monitoring volatile organic compounds in ambient air with optical spectroscopy[J]. Spectroscopy and Spectral Analysis, 2009, 29(12):3219-3203(in Chinese). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=gpxygpfx200912008
[2] LIN W H, GAO Z H, YANG Y, et al. NO2 detection based on laser spectrum differential method[J]. Laser Technology, 2014, 38(6):835-838(in Chinese). http://d.old.wanfangdata.com.cn/Periodical/jgjs201804001
[3] HE Y, ZHANG Y J, KAN R F, et al. Open-path online monitoring of ambient atmospheric CO2 based on laser absorption spectrum[J]. Spectroscopy and Spectral Analysis, 2009, 29(1):10-13(in Chinese). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=gpxygpfx200901003
[4] SUN K, XIE L, JU Y, et al. Compact fiber-optic diode-laser sensor system for wide-dynamic-range relative humidity measurement[J]. Chinese Science Bulletin, 2011, 56(32):3486-3492. DOI: 10.1007/s11434-011-4710-x
[5] YANG B, HE G Q, LIU P J, et al. Research progress of tunable diode laser absorption spectroscopy for combustion diagnostics[J]. Laser Technology, 2011, 35(4):503-510(in Chinese). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=jgjs201104016
[6] QU J M, FENG X Q, LIU Y C, et al. Source characteristics of VOCs emissions from vehicular exhaust in the pearl river delta region[J]. Acta Scientiae Circumstantiae, 2014, 34(4):826-834(in Chinese). http://d.old.wanfangdata.com.cn/Periodical/hjkxxb201404004
[7] WANG Y T, LIU J, ZHANG J C, et al. A methane gas sensor with optic fiber based on frequency harmonic detection technique[J]. Measurement and Control Technology, 2003, 22(11):19-21(in Chinese).
[8] SKELDONA K D, McMILLANB L C, WYSE C A, et al. Application of laser spectroscopy for measurement of exhaled ethane in patients with lung cancer[J]. Respiratory Medicine, 2006, 100(2):300-306. DOI: 10.1016/j.rmed.2005.05.006
[9] LI M X, LIU J G, KAN R F, et al. Design of real-time measurement of atmospheric CO and CH4 based on tunable diode laser spectroscopy system[J]. Acta Optica Sinica, 2015, 35(4):0430001(in Chinese). DOI: 10.3788/AOS
[10] HARRISON J J, BERNATH P F. Infrared absorption cross sections for propane(C3H8) in the 3μm region[J]. Journal of Quantitative Spectroscopy & Radiative Transfer, 2010, 111(9):1282-1288.
[11] SONG S F, XING W R, LIU M. Theory and research advancement of quantum cascade lasers[J]. Laser & Infrared, 2013, 43(9):972-976(in Chinese). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=jgyhw201309002
[12] LIU J Q, ZHAI S Q, KONG N, et al. Quantum cascade infrared photodetectors[J]. Inflared and Laser Engineering, 2011, 40(8):1397-1402(in Chinese). http://d.old.wanfangdata.com.cn/Periodical/hwyjggc201108001
[13] CHAN K, ITO H, INABA H. All-optical remote monitoring of propane gas using a 5-km-long, low-loss optical fiber link and an InGaP light-emitting diode in the 1.68μm region[J]. Applied Physics Letters, 1984, 45(3):220-222. DOI: 10.1063/1.95189
[14] XIN Z. Diode-laser absorption sensors for combustion control[D]. Stanford, USA: Stanford University, 2005: 5-22.
[15] GENG H, ZHANG Y J, LIU W Q, et al. Acquisition method of high resolution spectra of ethanol vapor in near-IR range[J]. Journal of Atmospheric and Environment Optics, 2012, 7(1):57-62(in Chinese). http://d.old.wanfangdata.com.cn/Periodical/dqyhjgxxb201201010
[16] SHARPE S W, JOHNSON T J, SAMS R L, et al. Gas-phase databases for quantitative infrared spectroscopy[J]. Applied Spectroscopy, 2004, 58(12):1452-1461. DOI: 10.1366/0003702042641281
-
期刊类型引用(3)
1. 崔子健,张庆才,谭晓茗,张靖周. 带同轴引气激光制孔中的气动特性分析. 南京航空航天大学学报. 2024(04): 658-667 .  百度学术
百度学术
2. 王海龙,李铁,王宏建. AlN陶瓷的飞秒激光螺旋制孔研究. 机械工程与自动化. 2021(06): 120-121+123 .  百度学术
百度学术
3. 王桂霞,崔智勇. 基于激光雷达的机器人精准制孔控制系统设计. 激光杂志. 2019(10): 103-106 .  百度学术
百度学术
其他类型引用(7)



 下载:
下载: