HTML
-
金刚石具有较好的结构与性能,人们常常将金刚石制作成工具来加工各种金属材料和硬脆性材料如陶瓷、蓝宝石等[1]。近年来,对于制造单层金刚石工具的方法一直备受关注,国内外主要的研究方法为真空炉中钎焊和感应加热钎焊[2-4],但这两种方法都有自身的不足。真空炉中钎焊的不足主要是因为生产效率一般,并且金刚石磨粒会长时间暴露在高温环境,从而使得金刚石磨粒热损伤严重,影响质量。高频感应钎焊的局限性主要是线圈设计制作比较复杂和加热范围受线圈形状影响[5-6]。而激光束能量易于精确控制、加热效率高等特点,使得激光钎焊过程中金刚石磨粒的热损伤和基体热变形得到较好控制。因此选用激光束进行钎焊的这种方法,不仅能够解决上述两种工艺中存在的不足,而且还能获得磨削性能良好的加工工具[7]。
HUANG等人[8]最早提出了激光钎焊金刚石工具,利用Cu基钎料激光钎焊金刚石磨粒制备工具,最终摩擦试验结果良好。YANG等人[9-10]使用Ni基合金钎料进行了激光钎焊试验,讨论了试验工艺和结合界面,利用制作的砂轮做磨削试验时,金刚石并无脱落。YANG等人[11]利用CO2激光器进行了试验,探讨了金刚石热损伤情况以及钎焊结合层的影响因素。HUANG等人[12]用光纤激光器进行钎焊时,使用的是铁基焊料,金刚石磨损状态在一定时间内保持相对稳定,有良好磨削性能。GUO等人[13]利用脉冲激光器对两种镍基合金钎料分别进行了钎焊试验,试验表明金刚石与两种焊料都有较好的润湿性。GUO[14]利用YAG固体激光器进行了钎焊,得到了良好的金刚石界面冶金反应,且磨削试验效果好,并无金刚石磨粒脱落。ZHAO[15]使用添加元素Ti和Al的Sn基活性钎料,进行了超声辅助钎焊蓝宝石,发现超声钎焊和超声热浸均可以改善润湿性。ZHAO[16]采用铝基钎料进行了超声辅助钎焊蓝宝石试验,分析了蓝宝石界面的反应机制,确认了超声波效应能够使表面反应层变厚。
激光钎焊金刚石的本质是在高温条件下,合金钎料中的活性元素如Cr, Ti等直接与金刚石的C反应,在金刚石表面生成具有金属性的化合物,利用这种与金刚石界面冶金结合的化合物来提高合金钎料对金刚石的润湿性和结合强度,称为金刚石表面金属化[17-19]。本文中基于第一性原理密度泛函理论,计算了激光钎焊界面冶金反应可能产生Cr3C2和Cr7C3的晶格常数、形成焓和结合能等参量,并探讨了Cr3C2和Cr7C3的结构稳定性以及力学性能。采用Ni-Cr合金钎料,借助光纤激光热源对金刚石磨粒进行了激光钎焊试验。采用扫描电镜(scanning electron microscope,SEM)、X射线能谱仪(X-ray energy dispersive spectro-scopy,EDS)和X射线衍射仪(X-ray diffraction,XRD)对金刚石磨粒与合金钎料结合界面的微结构以及金刚石磨粒表面碳化物进行了详细分析。
-
在镍基钎料中,通过引入Cr元素来提高金刚石与钎料基体的润湿性,实际上是金刚石表面C与镍铬合金的Cr在结合界面处直接反应,然后在磨粒表面形成一层结构稳定碳化铬,来保证界面处有良好的冶金结合。
本文中采用广义梯度近似(general gradient a-pproximation, GGA)的Perdew-Burke-Ernzerhof(PBE)方法,利用商业软件CASTEP(cambridge serial total energy package)模块求解Kohn-Sham方程计算Cr3C2和Cr7C3两种化合物的电子结构和性能。势函数使用倒易空间中超软赝势,体系平面波截断能设定为500eV[20]。将自洽场计算总能量的收敛精度设定为1×10-6eV,作用在各原子上的力小于0.002eV/nm。布里渊区的K点对于Cr3C2和Cr7C3分别采用6×10×3与6×4×2的Monkhorst-Pack网格划分[21],图 1为优化后的两种碳化铬晶体结构。
表 1中为两种碳化铬晶格常数(a, b, c)、形成焓ΔH和结合能Ec的计算值。其结果与相关文献[21]中的数据相吻合,表明所选的计算参量是可靠的。结合能是指几个粒子从自由状态结合成为一个复合粒子时所释放的能量。形成焓是指一定温度下,各元素从其单质状态反应生成1mol化合物的热效应。结合能数值大小与晶体的结构稳定成正比关系,形成焓数值大小与晶体的热力学稳定性成反比关系。根据表 1中的计算结果可知,Cr3C2的结合能数值要比Cr7C3的结合能数值大,并且Cr3C2形成焓数值比Cr7C3生成焓数值小。因此,Cr7C3的结构稳定性要强于Cr3C2,Cr3C2的热力学稳定性要优于Cr7C3。
phases a/nm b/nm c/nm ΔH/(kJ·mol-1) Ec/(kJ·mol-1) Cr3C2 0.547 0.279 1.147 -17.307 -859.581 Cr7C3 0.453 0.698 1.198 -16.346 -867.273 Table 1. The calculated cell parameters, formation enthalpy and cohesive energy of two chromium carbides
表 2中为Cr3C2和Cr7C3两种碳化铬的力学性能参量, 包括体模量B、剪切模量G、杨氏模量E和泊松比ν,这些参量是通过广义胡克定律应力关系计算出来的。由表 2可知,两种碳化铬的泊松比分别为0.28和0.35,由于两者的值都与纯金属材料的泊松比(0.3)十分接近,充分表明Cr3C2和Cr7C3是具有金属性质的化合物。体模量与剪切模量的比值(B/G)能体现化合物延展性的好坏,其临界值是1.75[22]。对于韧性材料,B/G值大于1.75;对于脆性材料,B/G值小于1.75。Cr3C2和Cr7C3的B/G值分别为1.90和2.56,显然两者都有较好的韧性,且Cr7C3化合物的韧性比Cr3C2强。
phases B/GPa G/GPa E/GPa ν B/G Cr3C2 316.3 166.1 423.9 0.28 1.90 Cr7C3 314.7 122.8 362.5 0.35 2.56 Table 2. Parameters of mechanical performance of two carbides
-
试验中采用的磨粒材料是黄河旋风股份有限公司生产的大小为35目/40目的HSCD90人造金刚石,金刚石磨粒原始表面形貌如图 2所示。基体为45#钢,尺寸规格为100mm×100mm×10mm;钎料是大小为200目的Ni-Cr合金粉末,组成成分如表 3所示。基体表面用高精度数控平面成型磨床进行抛光加工,并用丙酮进行清洗。首先将镍基合金粉末和金刚石磨粒依次置于钢板上,然后将试件放入由光纤激光器、超声波发生装置、气体保护装置和数控加工机床组成的超声辅助激光钎焊试验平台中进行试验,如图 3所示。
Cr Fe Si B Ni 0.080 0.025 0.045 0.030 balance Table 3. Chemical component (mass fraction w) of Ni-Cr alloy filler
钎焊完成后,采用线切割切下小试样,然后打磨、抛光、腐蚀处理,再利用TESCAN MIRA3 LMU场发射扫描电镜系统观察钎焊后样品表面和钎焊横截面形态,以及腐蚀后金刚石磨粒形貌;通过Oxford X-Max20能谱系统线扫描分析了金刚石与钎料结合横截面;利用理学D/MAX-Rapid X射线衍射仪研究了金刚石与钎料界面冶金反应的生成物结构。
-
图 4为金刚石磨粒与钎料合金结合界面形貌及其元素分布。从图 4可发现, Ni, Cr, C 3种元素在金刚石与钎料熔合区之间都有比较明显的过渡现象,其中能清楚地看到金刚石界面附近Cr元素含量较高,表明Cr元素在结合界面产生了一定富集。此外,过渡层的厚度大约为4μm。
-
图 5为钎焊金刚石磨粒表面形貌SEM图。很明显, 磨粒的表面有形状规矩的化合物生成;出露的金刚石磨粒原始棱角与平整表面仍可见,说明金刚石磨粒与钎料合金间发生了化学冶金反应且金刚石基体基本结构未发生变化。进一步观察发现,金刚石磨粒表面生成物主要呈条状和片状,分布整齐有规律,与金刚石有位向关系,造成这一现象的原因主要是与金刚石表面上碳原子的排列有关[23],如图 6所示。

Figure 6. Morphology of products on surface of diamond(square zone in Fig. 5)
图 7为激光钎焊和超声辅助激光钎焊后金刚石磨粒XRD谱图。由图 7a可知,激光钎焊的金刚石磨粒表面的特征峰主要为Cr3C2衍射峰,说明激光钎焊金刚石磨粒表面发生了金属化反应,并且表面金属化反应层的产物是Cr3C2化合物。由图 7b可知,在超声辅助激光钎焊下特征峰主要为Cr3C2与Cr7C3衍射峰,说明超声辅助条件表面金属化反应层的生成物为Cr3C2与Cr7C3两种化合物。结合前述计算结果,超声辅助激光钎焊金刚石的方法不仅可以促进使金刚石磨粒和镍铬钎料之间的界面冶金反应,提高润湿性,且生成了韧性更好的Cr7C3化合物。
-
图 8为金刚石磨粒与Ni-Cr合金钎料界面冶金反应过程示意图。
在一定的条件下,扩散变化的活性Cr元素和C元素能发生多相反应,当碳含量在13%和9%时会分别发生以下反应[24]:
式中, ΔGm为浇铸温度,T为开尔文温度。从上述两个反应式以及金刚石表面金属化分析可以看出,激光金刚石界面冶金反应层的结构主要是由活性C原子与Cr原子的相对量决定。在钎焊试验中,激光能量提供表面金属化反应条件,反应所需C原子由金刚石表面提供,而Cr原子则由Ni-Cr合金钎料中Cr粉提供,如图 8a所示。在反应初期,金刚石磨粒表面的C原子数量多,由于激光的速冷速热,所能给予化合反应的时间短,Ni基合金钎料熔池中的大多数Cr原子无法在短时间内向结合界面扩散,又由于界面处的C原子浓度高,所以此时界面冶金反应层结构为高含碳量的Cr3C2,如图 8b所示。通过在激光钎焊过程中引入超声,在反应后期,界面冶金反应在金刚石表面已经消耗了大量C原子,导致表面C原子的减少。超声[25]能使熔融态合金钎料产生大量空泡,由于空泡之间的相互作用,使熔融态合金钎料与金刚石磨粒界面处产生局部的高温高压,它可以为合金钎料熔池中的Cr原子提供能量,使Cr原子向结合界面扩散,并增加界面处Cr原子的相对量,又由于超声的作用使空泡破裂,能增大金刚石磨粒与熔融态合金钎料非均相界面面积,加速化学反应。所以在反应的短时间内,当Cr3C2层生长到一定厚度后,生成含碳量少的Cr7C3,如图 8c所示。因此随着界面冶金反应持续进行,界面反应层结构从内向外依次是Cr3C2, Cr7C3碳化物。
2.1. 试验方法
2.2. 金刚石磨粒与Ni-Cr合金钎料界面元素分布特征
2.3. 金刚石磨粒表面金属化反应层分析
2.4. 界面冶金反应机理
-
(1) 通过第一性原理计算并分析了Cr7C3和Cr3C2两种碳化铬的结构稳定性与力学性能,发现两者都是热稳定结构,且Cr7C3比Cr3C2的韧性更好,为金刚石表面金属化提供了理论依据。
(2) 采用Ni-Cr合金钎料对金刚石磨粒进行激光钎焊,可以实现金刚石磨粒的表面金属化,试验中发现, 光纤激光钎焊后的金刚石磨粒与Ni-Cr合金钎料存在界面冶金反应层,厚度约为4μm,金刚石磨粒表面生成物呈片状和条状形态。由XRD分析发现,光纤激光钎焊过程中金刚石磨粒表面生成物主要为Cr3C2,超声辅助光纤激光钎焊过程中金刚石磨粒表面生成物为Cr3C2和Cr7C3。
(3) C-Cr化学冶金反应分析表明,激光钎焊过程中金刚石磨粒与Ni-Cr合金钎料界面首先在靠近金刚石表面生成含碳量高的Cr3C2;随着界面冶金反应的持续进行,钎料中Cr原子向金刚石一侧扩散,继而生成含碳量低的Cr7C3。

 Map
Map

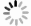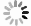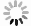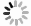







 DownLoad:
DownLoad: