HTML
-
激光诱导击穿光谱(laser-induced breakdown spectroscopy,LIBS)是一种元素分析的新技术,对几乎所有物质形态(诸如气溶胶、岩石、金属、骨骼、血液、生物组织以及聚合物等)的靶样都可以进行元素分析,其定性、定量分析是通过检测等离子体(高能激光脉冲聚焦烧蚀物质产生)发射光谱来实现。LIBS技术在各种实验条件下都可以开展实施,包括常压、真空、海洋深处、或者是极端恶劣的环境中(如反应堆), 其具有响应时间短、样品无需制备或仅需简单制备、可免校准方法和多元素同时在线分析等优点,已广泛应用于元素分析领域[1-3]。基于此,国内外学者日益关注对LIBS的研究,这项技术也逐步从实验室研究推广到生物医学、太空检测、环境监测、资源探测、反应堆乏燃料以及放射性同位素分析等诸多领域[4-10]。目前,LIBS开展特定样品的元素成分分析,探测灵敏度多为mg/L量级,一直以来, 众多科学家关注的焦点是如何极大地降低其检测限,有效地提高LIBS信号强度。
表面等离激元共振是纳米结构具有的特性之一,很大程度上能增强局域电场[11-12],对LIBS信号增强效果显著,信号强度能达到数个量级的增强。纳米颗粒增强LIBS(nanoparticle enhanced LIBS, NELIBS)只需要简单的优化制样方法,不需要对实验设备进行复杂的改进, 即可对LIBS信号进行增强。意大利GIACOMO课题组对NELIBS展开了系统且具有重要影响力的研究,比如通过使用纳米银溶胶将其滴加到金属、半导体和绝缘体表面,降低了固体靶的烧蚀阈值,对金属靶样的信号强度增强达到了1~2个数量级[13]。基底在滴加样品溶液前沉积一层纳米金颗粒,使得检测限下降到mg/L以下[14]。LIU等人使用金属螯合物诱导金纳米粒子聚集,检测水中重金属元素(Cd, Cu, Ag, Pb和Cr),检测限达到μg/L量级[15];该课题组还提出了在多孔静电纺超细纤维上添加纳米粒子涂层的方法增强水体系中金属离子的LIBS信号[16]。LIU等人从纳米颗粒对信号强度、信号噪声比和相对标准差的影响出发,特别注意了预烧蚀对纳米颗粒增强效果的影响以及NELIBS很好的可重复性,从而促进了对NELIBS的全面了解[17]。
作者所在课题组引入两亲性分子到NELIBS,使得信号检测限达到μg/L量级[18]。使用磁控溅射方法,研究了纳米团簇对NELIBS信号的影响[19]。另外在纳米银溶胶中加入不同浓度的氯离子后,利用透射电镜观察纳米颗粒的状态,并结合专业微纳光子学仿真分析软件计算纳米粒子不同的状态对局域电磁场增强的影响[20]。基于此,有必要对卤素离子做进一步的研究。本文中将分析不同种类的卤素离子对NELIBS信号的影响。
-
使用Lee-Meisel法[21]制备灰绿色的纳米银溶胶(灰银胶),即柠檬酸钠(C6H5Na3O7)还原硝酸银(AgNO3)生成银纳米粒子。
在超薄碳膜上滴加银胶溶液,使用透射电子显微镜对样品进行形貌表征。紫外-可见分光光度计用来测量银胶的吸收光谱[22]。
-
配制以下溶液:浓度为0.01 mmol/L十二烷基硫酸钠(sodium dodecyl sulfate,SDS)(质量分数不小于99.0%)溶液[20];浓度为2 mmol/L的MgSO4(质量分数不小于99.0%)溶液;浓度为0.01 mmol/L、0.05 mmol/L、0.1 mmol/L、0.15 mmol/L、0.2 mmol/L、0.3 mmol/L、0.4 mmol/L、0.5 mmol/L、1 mmol/L、2 mmol/L、4 mmol/L和20 mmol/L的KBr(质量分数为99.9%)溶液;浓度为0.2 mmol/L的KCl(质量分数不小于99.99%)溶液;浓度为0.05 mmol/L、0.1 mmol/L、0.15 mmol/L、0.2 mmol/L、0.3 mmol/L、0.4 mmol/L、0.5 mmol/L、1 mmol/L、2 mmol/L和4 mmol/L的KF(质量分数为99.9%)以及KI(质量分数不小于99.9%)溶液。
依次用去离子水和无水乙醇超声清洗9 cm2正方形单面抛光硅片(P型硅,100晶向),20 min后干燥使用[22]。
-
采用Nd ∶YAG纳秒脉冲激光器(波长为532 nm、单脉冲能量80 mJ),纳秒激光聚焦烧蚀靶样产生等离子体。等离子体发射光谱(侧向收集)聚焦到光纤上,经光纤耦合传导到光谱仪中进行测量。光谱仪延迟时间、积分门宽和信号增益分别设置为0.4 μs、5 μs和1500,采集单发烧蚀LIBS信号,多次测量以降低误差[22]。
1.1. 纳米颗粒制备及表征
1.2. 样品制备
1.3. LIBS实验
-
首先对Ag纳米粒子(nanoparticles,NPs)进行透射电子显微镜(transmission electron microscope,TEM)表征,形貌表征结果如图 1所示。TEM测试图(见图 1a, 图 1c, 图 1e)和尺寸统计分布直方图(见图 1b, 图 1d和图 1f)分别对应的是10 mL、5 mL、2 mL柠檬酸钠溶液(质量分数为1%)和2 mL的AgNO3溶液(质量分数为1%)制备的灰银胶。从图 1中可以看出,减少柠檬酸钠的用量会导致Ag NPs直径变大(平均直径为34.53 nm,39.65 nm,59.01 nm)。
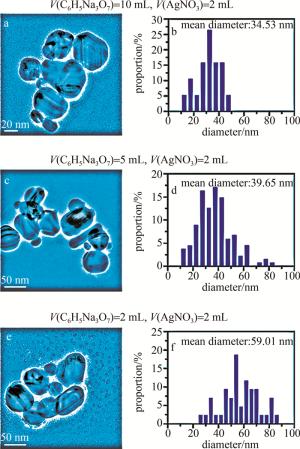
Figure 1. TEM images and size distribution statistics of different volumes of C6H5Na3O7 solution (mass fraction of 1%) and 2 mL AgNO3 solution (mass fraction of 1%) for preparation of grey silver sol
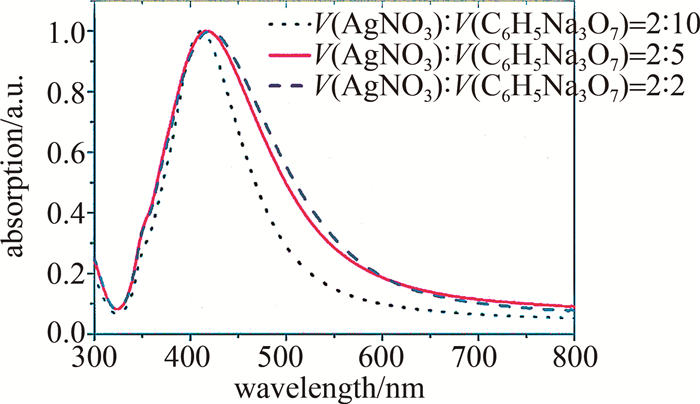
图 2是灰银胶吸收光谱的测量结果。可以看到,减少柠檬酸钠的用量会使420 nm附近的共振吸收峰红移。由于较小尺寸的胶体会更加稳定,不易团聚,并且为尽量使其吸收峰位更接近532 nm激光[22],在之后的实验中选择了纳米粒子平均直径为39.65 nm的灰银胶。
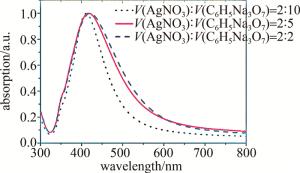
Figure 2. Absorption spectra of different volume ratios of AgNO3 solution (mass fraction of 1%) and sodium citrate solution (mass fraction of 1%) for preparation gray silver sol
以氯离子为例, 研究了卤素离子对银纳米溶胶聚集效应的影响,发现不同浓度的氯离子诱导银纳米颗粒聚集的程度不同,随着氯离子浓度的增加,银纳米粒子逐渐团聚,间距越来越小,直至凝聚沉淀[20]。
图 3为加入氯离子浓度为20 mmol/L时的TEM图。可以看到, 纳米颗粒团聚十分严重,出现链状的凝聚,颗粒几乎很难分辨。
-
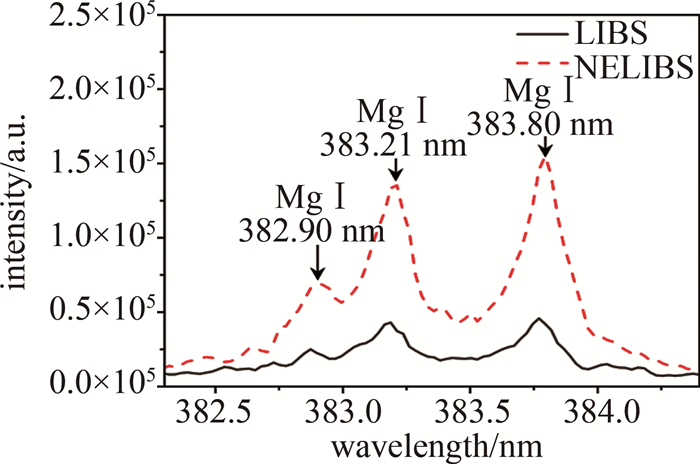
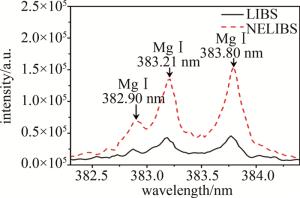
由作者团队之前的工作[20]可知,氯离子可以引起纳米颗粒团聚现象,0.2 mmol/L的氯离子NELIBS信号的程度最大。基于此,用同样的方法研究了不同种类的卤素离子对NELIBS信号的影响。在MgSO4溶液中加入KF、KBr和KI溶液,引入卤素离子后测量NELIBS信号的变化。图 4是靶元素Mg的一个典型的光谱图。光谱范围在382.3 nm~384.4 nm,382.90 nm、383.21 nm、383.80 nm是Mg元素的3个特征峰。从图中可以清楚看到, 添加纳米颗粒之后的LIBS信号有明显的增强效果。
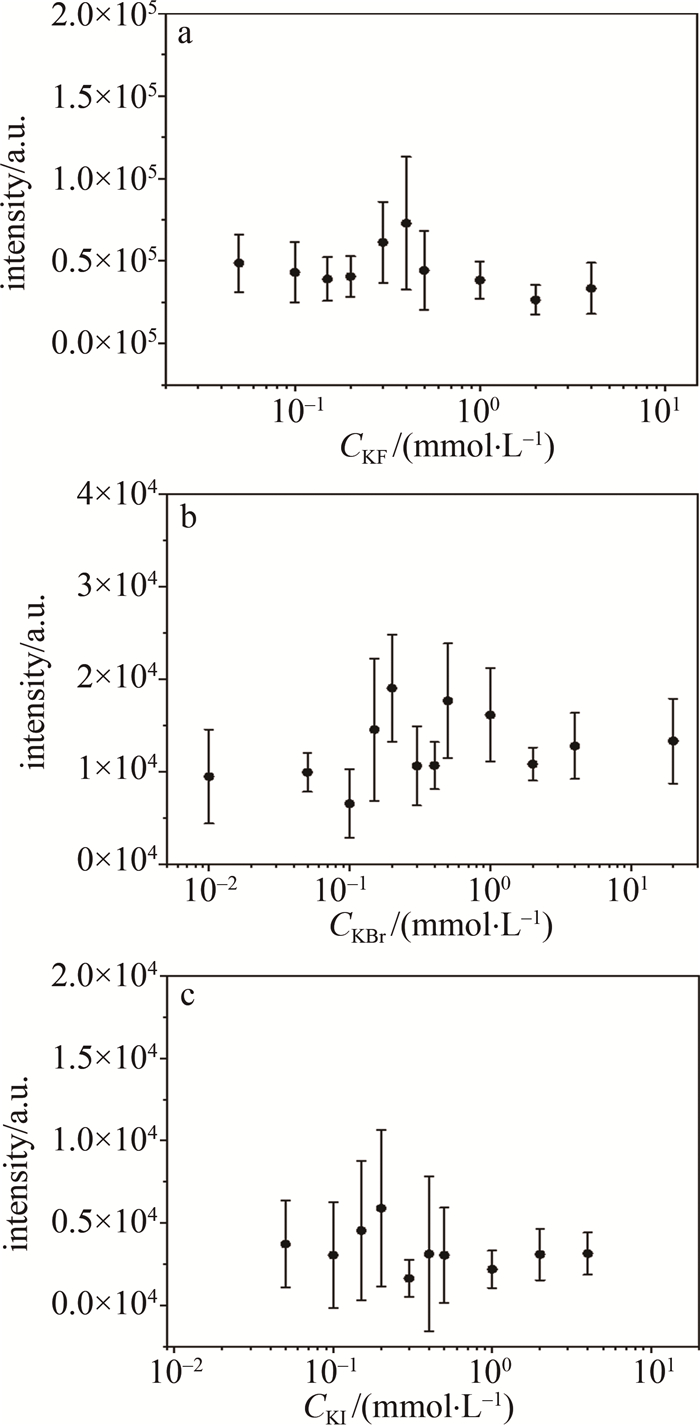
改变卤素离子浓度,测量了SDS+Ag NPs MgSO4(2 mmol/L)样品中Mg元素的NELIBS信号强度,实验结果如图 5所示。图中信号强度是通过扣除本底信号后选取382.66 nm、384.13 nm为左右端点进行面积积分,将积分值作为信号强度。当Br-、I-浓度为0.2 mmol/L时Mg的NELIBS信号达到最强,与之前工作[20]中Cl-实验结果一致,而F-浓度为0.4 mmol/L时Mg的NELIBS信号最强。
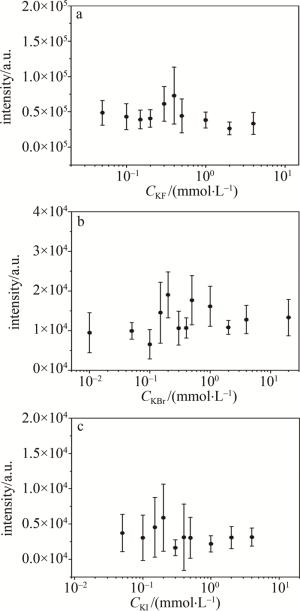
Figure 5. NELIBS signal of Mg changes when different concentrations of KF, KBr and KI are added into 2 mmol/L MgSO4 solution
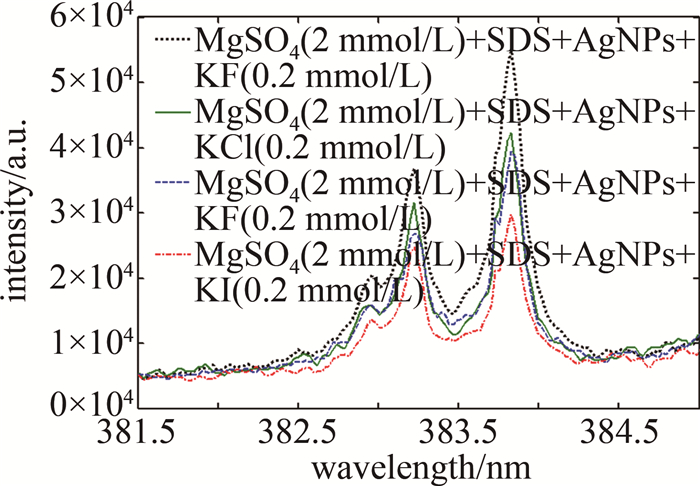
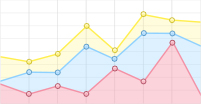
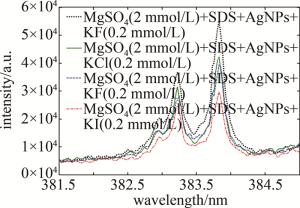
基于图 5中的实验结果,综合考量实验因素,固定卤素离子的浓度为0.2 mmol/L,研究分析了4种卤素阴离子对NELIBS信号强度的影响,结果见图 6。从图 6中可以看出, 分别加入KF、KCl、KBr、KI溶液(浓度均为0.2 mmol/L)到2 mmol/L MgSO4+0.01 mmol/L SDS+Ag NPs的溶液中,当银溶胶中加入0.2 mmol/L卤化钾时,Mg的NELIBS信号得到了不同程度的增强,卤素离子对Mg的NELIBS信号的增强效果为:F->Cl->Br->I-。
2.1. 银纳米粒子的表征
2.2. LIBS谱线特征
-
研究了不同种类、不同浓度的卤素阴离子对Ag NPs增强LIBS信号的影响。在添加卤素离子之后对比分析了Mg的NELIBS信号,发现其信号会得到不同程度的增强,结合之前氯离子的工作,表明卤素阴离子与Ag NPs之间存在着一定的相互作用。Ag NPs具有较大的比表面积,少量未反应的银离子可能会吸附在Ag NPs的表面,而银离子与卤素阴离子具有库伦相互作用,使得卤素阴离子在Ag NPs的表面上吸附,引起Ag NPs的团聚现象,从而使得Ag NPs之间的距离发生了显著的变化;F-对Ag NPs的影响比Cl-、Br-、I-要强,F-与Ag NPs的活性点结合更容易,原因可能是和F、Cl、Br、I的原子序数有较大的关系。卤素阴离子对Mg的NELIBS信号增强的作用为:F->Cl->Br->I-。

 Map
Map



 DownLoad:
DownLoad: