HTML
-
先驱体转化陶瓷(polymer drived ceramics,PDC)法是首先将聚合物先驱体经过成形、交联,而后在高温条件下裂解先驱体并结晶制备陶瓷材料的一种工艺方法。目前PDC法已经广泛应用于制备陶瓷涂层的研究领域内[1-2],PDC法中陶瓷相经原位合成,与基体具有优异的结合强度,且具有优异的力学、电化学和摩擦学等性能,而广泛应用于金属表面的防腐蚀、抗高温氧化及抗磨损等领域[3-7]。GOERKE等人在1000℃惰性气体氛围中进行热裂解聚硅氧烷先驱体,在材料表面制备了0.2μm~3μm的非晶态SiOC陶瓷层,研究表明陶瓷涂层极大地提升了基底的防氧化性[8]。但是经PDC法制备的陶瓷材料中往往存在较多孔隙和裂纹,这是由于先驱体在陶瓷化过程中会出现体积收缩和密度变化。因此,常采用添加活性填料制备有机高聚物填料体系,通过热裂解生成新的陶瓷相来填补因体积收缩产生的孔隙,以降低所制备陶瓷涂层的孔隙率和裂纹[9]。ERNY等人以添加了Ti和MoSi2活性填料聚硅氧烷为先驱体体系,在加热温度超过800℃条件下制备了含TiC陶瓷相的复相陶瓷,发现其弯曲强度可达到330MPa[10]。但由于活性填料在先驱体难以分散均匀,导致生成的陶瓷涂层组成分布不均匀。为了解决活性填料在先驱体难以分散均匀的问题,通过制备含金属元素的有机硅聚合物先驱体进行高温裂解制备涂层,金属元素有机硅先驱体可以经化学反应生成新生陶瓷相,提升陶瓷产率,赋予陶瓷材料一些特殊功能,同时避免了金属粉末的分散问题[11-12]。MOTZ等人通过高温裂解涂覆在金属材料表面的经金属钛有机化合物改性的聚硅氮烷先驱体涂层,制备了与金属基体结合性能十分优异的陶瓷涂层[13]。近年来,人们开始用高能激光作为热源裂解聚合物先驱体制备陶瓷涂层,克服了热裂解制备涂层材料时间周期长的不足,如XUE等人用CO2激光裂解液态硅氧烷先驱体(polydimethylsiloxane, PDMS)制备出由非晶态SiO2、晶态SiC组成的陶瓷涂层[14]。FRIEDELA等人采用选择性激光固化(selective laser curing,SLC)对工件表面常温下为固态粉末的聚甲基硅倍半氧烷(polymethylquisiloxane, PMS)进行裂解制备了SiOC陶瓷涂层,发现工件力学性能有明显提升[15]。但激光裂解聚合物先驱体制备陶瓷涂层的反应过程、作用机理等很多方面并不清楚。本文中对激光裂解钛酸四丁酯改性硅氧烷先驱体所制备陶瓷涂层的组成、结构进行了表征,初步研究了其形成机理。
-
试验中所用聚硅氧烷先驱体为聚二甲基硅氧烷(PDMS,化学纯),其分子量为770~13900、相对密度为0.94,其分子式为(—Si(CH3)2—O—)n。钛酸四丁酯为工业纯,其分子式为Ti(OC4H9)4。
钛酸四丁酯改性聚硅氧烷的方法如下:按4:1质量分数分别称取80g聚二甲基硅氧烷和20g二甲苯后,放入容器中超声混合5min~10min,然后称取不同质量的钛酸四丁酯缓慢滴入聚二甲基硅氧烷和二甲苯的混合溶液中,并继续超声分散5min左右,获得分散均匀质量分数分别为0.05, 0.10, 0.15和0.20的钛酸四丁酯改性聚二甲基硅氧烷混合溶液。
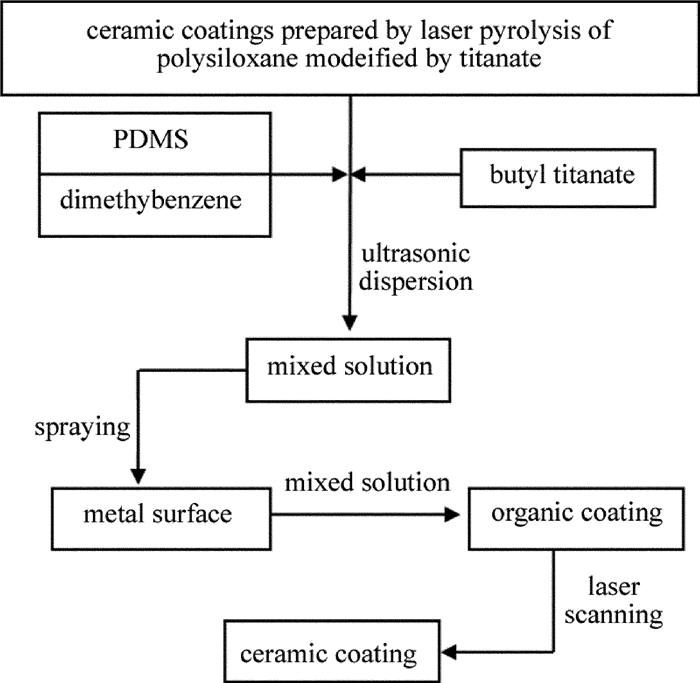
激光裂解钛酸四丁酯改性聚二甲基硅氧烷制备陶瓷涂层的方法:将钛酸四丁酯改性聚二甲基硅氧烷混合溶液均匀喷涂到清洁的45#钢表面,80℃下固化1.0h,获得表面平整、厚度约为0.4mm~0.5mm的透明有机涂层。将该有机涂层放入氩气保护的装置内,采用大族激光科技有限公司生产的CST2200型CO2连续高能激光扫描裂解有机涂层,其中激光波长为1.06μm、激光功率为900W、扫描次数为2次、扫描线速率为14mm/s、扫描路径为S型。激光裂解结束后在空气中自然冷却到室温,即可获得陶瓷涂层,其工艺流程简图如图 1所示。

Figure 1. Process flow chart for the preparation of ceramic coatings by laser pyrolysis of polysiloxane modified by butyl titanate
采用激光裂解钛酸丁酯改性聚硅氧烷制备陶瓷涂层,其工艺流程主要包括先驱体体系制备-涂覆-固化-裂解-结晶。相对于PDC法的成形-交联-裂解-结晶过程所需较长制备周期而言[16],激光裂解先驱体制备陶瓷涂层的过程大幅度减少了先驱体体系裂解所需时间,其裂解时间主要取决于激光的扫描速率及扫描次数,高能激光的快速注入在极短时间内为先驱体陶瓷化提供了反应条件,使其迅速加热裂解,生成陶瓷涂层。
用Nova NanoSEM50型扫描电子显微镜(scanning electron microscope, SEM)分析陶瓷涂层的表面形貌,用DX-2700型X射线衍射仪(X-ray diffraction, XRD)分析陶瓷涂层物相组成,用K-Alpha型X射光电子能谱仪(X-ray photoelectron spectroscope, XPS)分析陶瓷涂层表面特征元素的化学价态,结合能测量精度为±0.1eV。
-
图 2是激光解不同质量分数w的钛酸四丁酯改性聚硅氧烷制备陶瓷涂层的SEM照片。可以看出,钛酸四丁酯的质量分数对激光裂解钛酸四丁酯改性聚二甲基硅氧烷制备陶瓷涂层的表面形貌有很大影响。当钛酸四丁酯的质量分数为0.00时,所制备的陶瓷涂层的表面堆积了大量絮状物,而且存在大量微小孔隙,见图 2a;当钛酸四丁酯质量分数为0.05时,涂层表面的絮状物和微小孔隙基本消失,出现大量的颗粒物,表面较为平整,见图 2b。钛酸四丁酯添加质量分数的不同对所制备陶瓷涂层表面陶瓷涂层表面形貌和颗粒物大小及孔隙都有较大影响,见图 2c~图 2e。
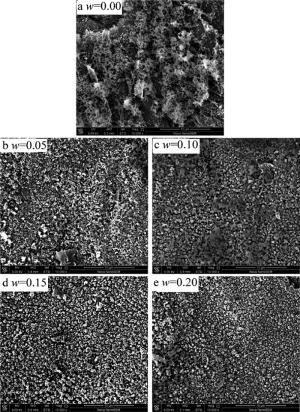
Figure 2. SEM images of ceramic coatings prepared by laser pyrolysis of polydimethylsiloxanes modified by titanyl titanat
图 3是激光裂解质量分数为0.05钛酸四丁酯改性聚二甲基硅氧烷所制备陶瓷涂层不同区域的能谱仪(energy dispersive spectroscopy,EDS)分析。其中区域Ⅰ中特征元素C,O,Si和Ti的相对含量分别为9.27%, 8.98%, 78.79%和2.96%,区域Ⅱ中特征元素C, O, Si和Ti的相对含量分别为9.45%, 9.33%, 77.97%和3.25%。可以看出,不同区域相同元素的相对含量基本接近,其中Ti元素的相对含量均在3%左右,说明Ti元素在涂层表面分布均匀,并未出现添加金属粉末时该元素在涂层表面出现的分散不均问题。
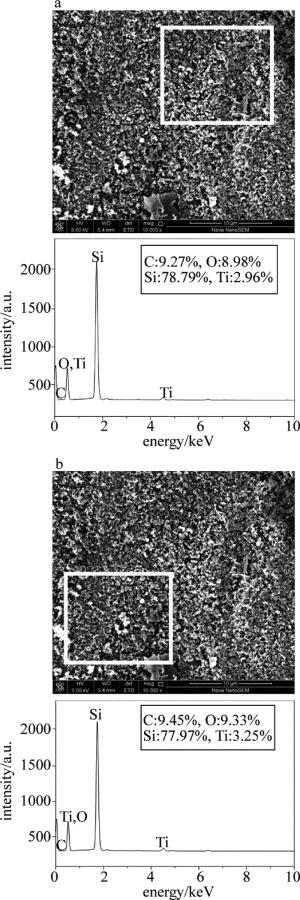
Figure 3. EDS analysis of different areas of ceramic coatings prepared by laser pyrolysis of polydimethylsiloxane modified by butyl titanate(mass fraction w=0.05)
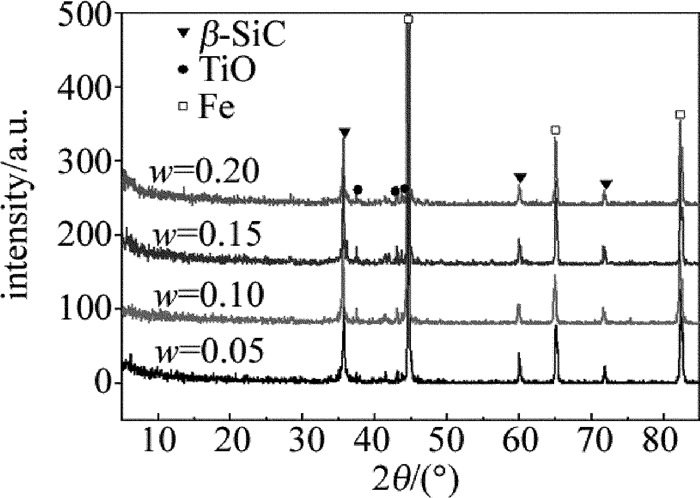
图 4中给出了激光裂解不同质量分数钛酸四丁酯改性聚硅氧烷制备的陶瓷涂层的XRD图。从图 4可以看出,激光裂解钛酸四丁酯改性聚二甲基硅氧烷获得的涂层中存在SiC,TiO2和Fe的衍射峰,说明涂层中含有晶态的β-SiC, TiO2和Fe。其中晶态β-SiC和TiO2的衍射峰是激光裂解钛酸四丁酯改性聚二甲基硅氧烷过程中的新产物引起的,而Fe的衍射峰是由45#钢基体引起的[14]。

Figure 4. XRD patterns of ceramic coatings prepared by laser pyrolysis of polydimethylsiloxanes modified by butyl titanate of different mass fraction
图 4在7.9°附近出现了TiO2-SiO2的弥散衍射峰,说明TiO2-SiO2以非晶态的形式存在。从图中还可以看出,随着钛酸四丁酯质量分数的增加,TiO2, TiO2-SiO2的衍射峰强度也在增强,说明涂层中含钛化合物的含量随钛酸四丁酯质量分数的增加而增加。
图 5是激光裂解钛酸四丁酯改性聚二甲基硅氧烷制备的陶瓷涂层表面特征元素的XPS解叠图谱。可以看出,激光裂解钛酸四丁酯改性聚二甲基硅氧烷制备的陶瓷涂层主要由SiO2, SiC, C6H18OSi2, TiO2和(TiO2)56(SiO2)44以及单质C等物质组成。
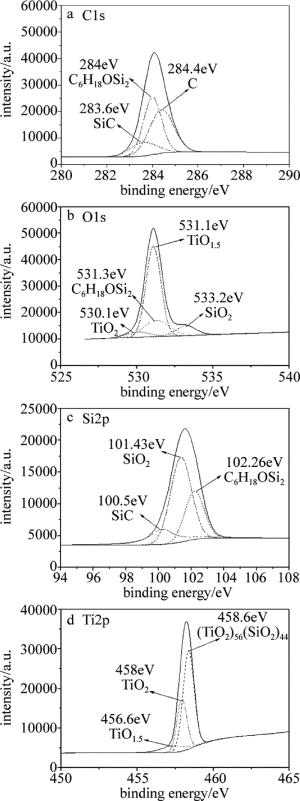
Figure 5. Curve-fitted XPS spectra of ceramic coatings prepared by laser pyrolysis of polydimethylsiloxanes modified by butyl titanate
由上述分析可知,除了激光裂解聚二甲基硅氧烷可生成晶态β-SiC、非晶态SiO2和单质C外[14],激光裂解钛酸四丁酯改性聚二甲基硅氧烷还生成了新的陶瓷相TiO2和(TiO2)56(SiO2)44,这些新的陶瓷相由于体积的增加对陶瓷涂层孔隙具有填补作用[9],因此随着钛酸四丁酯质量分数的增大,陶瓷涂层表面变得更为平整,孔隙逐渐减少。
钛酸四丁酯改性聚二甲基硅氧烷在高能连续激光作用下,激光与钛酸四丁酯改性聚二甲基硅氧烷发生非平衡态化学反应。由于聚二甲基硅氧烷中Si—O(422.5kJ/mol)的键能比C—Si(334.7kJ/mol)的键能高,在激光作用下C—Si键首先断裂,生成CH3自由基,在激光粒子的继续作用下,CH3自由基有一部分可以进一步生成碳自由基和H自由基,Si—O键断裂生成Si和O自由基。
这些自由基与自由基之间可能发生下列反应:
而钛酸四丁酯在高能粒子的作用下发生下酯化列反应:
新生的TiO2与通过自由基反应生成的SiO2发生混融,生成(TiO2)56(SiO2)44。
因此,钛酸四丁酯改性聚二甲基硅氧烷在高能连续激光作用,生成了气态的CO2, CO和H2,固态的SiO2, SiC, C6H18OSi2, TiO2, (TiO2)56(SiO2)44等物质。反应方程式如下:
-
(1) 激光裂解钛酸四丁酯改性聚二甲基硅氧烷制备的陶瓷涂层主要由晶态的SiC, TiO2、非晶态SiO2, (TiO2)56(SiO2)44单质C和C6H18OSi2组成,陶瓷涂层中含钛化合物的含量随钛酸四丁酯质量分数的增加而增加。
(2) 激光裂解钛酸四丁酯改性聚二甲基硅氧烷过程中生成的TiO2, (TiO2)56(SiO2)44等陶瓷相对陶瓷涂层孔隙具有填补作用。随着钛酸四丁酯质量分数的增大,陶瓷涂层表面变得更为平整致密,孔隙逐渐减少,涂层表面的颗粒物逐渐变小。
(3) 钛酸四丁酯改性聚二甲基硅氧烷在激光作用下发生非平衡态化学反应,聚二甲基硅氧烷在激光作用下主要发生自由基反应,而钛酸四丁酯在激光作用下主要发生酯化反应。

 Map
Map

 DownLoad:
DownLoad: