HTML
-
近年来,空气质量[1-2]越来越差,严重影响了人们的生活水平。小到家庭装修、汽车尾气[3],大到工业废气[4-5]、化工生产[6]、农业生产过程中都会产生很多污染气体,其中具有吸附性的NH3是主要污染气体之一。NH3是无色有刺激性气味,对人的皮肤、呼吸道有一定刺激或腐蚀,对人体的心血管神经和免疫系统都会造成极大危害,严重时甚至会导致死亡。另外,NH3气体与酸类反应产生的铵盐形成的气溶胶[7],会增加PM2.5颗粒物的浓度,必定会影响空气质量、大气能见度等,因此,研究出检测NH3气体浓度的高灵敏检测技术迫在眉睫。
吸收光谱技术分辨率高,可以实时、在线检测,依据这些优势,人们开始不断发掘其在大气环境监测方面的潜质,大功率可调谐激光的出现,也解除了其由于光源、功率密度等问题在应用上的限制。随着光谱技术的不断进步,人们通过采用不同技术手段对NH3浓度进行了测量,分别得到了相应的探测灵敏度[8]。1992年,FEHÉR等人采用1.5μm的半导体激光器作为光源,对NH3气体进行检测,得到探测灵敏度为1.5179mg/m3 。2003年,WEBBER等人为了提高激光光源功率,将掺饵光纤放大器与可调谐半导体激光器进行串联,得到NH3的检测灵敏度为0.0046mg/m3[10]。2006年,BESSON等人采用相同的方法[11],报道了基于近红外1532nm附近的氨气光声光谱检测系统。2011年,LINS等人[12]用高精度吸收光谱法分别对15NH3和14NH3在6468cm-1~6692cm-1范围内的吸收线进行了检测,并给出了14NH3和15NH3对应中心波长的气体分子吸收谱线的线强度S,压力增宽系数γb。2008年至2012年间,大连理工大学的PENG等人组成的光声光谱检测技术团队对人体呼出的NH3进行了研究[13],针对呼气中高浓度CO2和H2O背景对超低浓度NH3测量的干扰问题,提出了呼出氨气高灵敏度检测的多组分气体测量算法,实现了高浓度CO2和H2O背景下的NH3气体的高灵敏度测量,极限检测灵敏度达到0.0121mg/m3。可以看出,随着科技的发展、新型光源的出现、微弱信号处理技术的提高,气体检测技术将有更大的研究空间、更广泛的应用领域。
腔增强吸收光谱技术[14](cavity enhanced absorption spectroscopy,CEAS)是近十几年来发展起来的一种新型直接吸收光谱技术,由于其实验装置简单易于操作、有效吸收光程长、探测灵敏度高、鲁棒性强等优点,得到了相当高的关注。目前,国内还没有应用腔增强吸收光谱技术测量极低氨气气体浓度的实例。本实验的目的是搭建腔增强吸收光谱装置,并用该装置对NH3的气体浓度进行测量,通过得到的谱线展宽与压强的关系以及吸收度与压强的关系,分析其检测NH3浓度的可行性。
-
直接吸收光谱技术的基本原理是入射光通过一定长度的气体池,由于待测物质的部分吸收,透射出来的光强度会有一定程度的减弱,假设光的散射可以忽略,那么透射光相对于入射光的衰减就被定义为吸收。通过记录透射光强随波长的变化曲线就可以得到样品的吸收光谱,用Beer-Lambert定律[15]表达可以写成:
式中,I为入射光强;I0为透射光强;L为气体池长度(单位为cm),也就是待测气体的有效吸收光程;α为吸收系数(单位为cm-1),所有的定量吸收光谱都是以Beer-Lambert定律为基础。在进行数据处理时,通常令吸收度A为:
式中,Leq表示腔内介质有效吸收长度。
腔增强吸收光谱中的谐振腔是由两块分别置于气体池两端的壁上平凹高反镜组成,故腔长即为气体池长度L。而L与腔镜的曲率半径r之间满足关系[16]:0 < L < r或r < L < 2r。当光腔调节好后,通过扫描腔长,将激光耦合到谐振腔内,使其在两腔镜之间多次来回反射,谐振腔的精细度越高,激光在腔镜间反射的次数越多。激光透过谐振腔的强度随时间t变化可以表示成[17]:
式中,It为透射光强;I0为入射光强;ν表示频率;τ(ν)则是谐振腔的衰荡时间:
式中,c表示光速;L表示谐振腔的腔长;|lnR|表示谐振腔的腔镜损耗和腔内介质的损耗之和,其中,谐振腔的损耗主要包括腔内介质的吸收损耗以及腔镜各种损耗;R为腔镜反射率。
由于腔增强吸收谱光谱技术中,所用的高反镜反射率R一般都在99%以上,所以|lnR| ≈(1-R)。那么,(4)式就可以写成:
从(5)式中可以看出,腔镜反射率R、腔长L及腔内介质的吸收系数α是影响谐振腔的衰荡时间τ(ν)的3个主要参量。如果用谐振腔的衰荡时间τ(ν)与光速c的乘积来表示腔内介质对光的实际吸收路径,则:
如果腔内介质的单程吸收损耗远远小于腔镜损耗,即αL≤1-R,则(6)式可以写成:
相对于传统的直接吸收光谱来说,当气体池长度相同时,腔增强吸收光谱的有效吸收路径[18]被扩大了1/(1-R)倍。如果腔镜反射率在99%以上,那么有效吸收路径会增加1000倍甚至10000倍,这是其它吸收光谱技术所不能比拟的,同时这也使得腔增强吸收光谱具有很高的探测灵敏度。
-
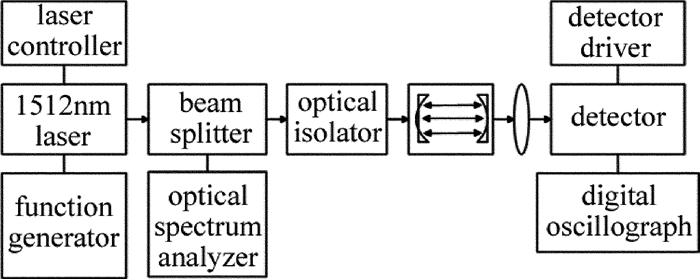
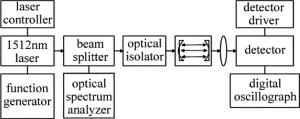
本实验中采用分布反馈式半导体激光器(NEL公司生产)作为光源。激光器由Semicon公司生产的LCM-6000型二极管激光电源驱动器控制。当激光器的工作温度设置为25.5℃、注入中心电流设置为85mA时,激光器输出的中心波长为6612.248cm-1。同时,设置函数发生器的频率、幅度、波形等参量,实现激光的波长扫描。输出的激光由光纤分束器(1: 9)分成两束,较弱的一束直接输入光谱分析仪(日本Advantest公司,Q8374型)测定激光波长,另一束入射到由两块平凹高反镜(凹面曲率半径为1m,腔镜反射率为99.9%)组成的长为34cm的高精密光学谐振腔。为避免半导体激光器的工作状态受到光学反馈的影响,需要在气体池前放置一个光纤隔离器(Thorlabs公司,IO-4-1480-VLP型)。透过谐振腔的激光经由一个透镜聚焦到InGaAs探测器(New Focus公司,2017型,在1.5μm附近的等噪声功率为1.3×10-13W·Hz1/2;探头供电电源为New Focus公司,0901型)上,将探测器接收到的光信号输入到数字示波器(Lecory公司,9310AM型),最后将波形数据导出进行数据处理。实验装置如图 1所示。
-
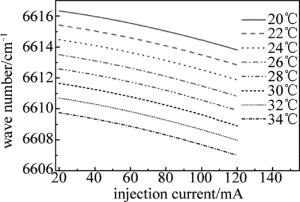
因为分布反馈式(distributed feedback,DFB)半导体激光器的工作波长与其工作温度和注入电流的大小有关,所以实验时,先设定激光器控制器的温度参量,然后通过函数发生器扫描电压改变其注入电流,实现波长扫描,最后,观察激光器的输出波长随注入电流的增加的变化情况,也就是对激光器进行波长定标。
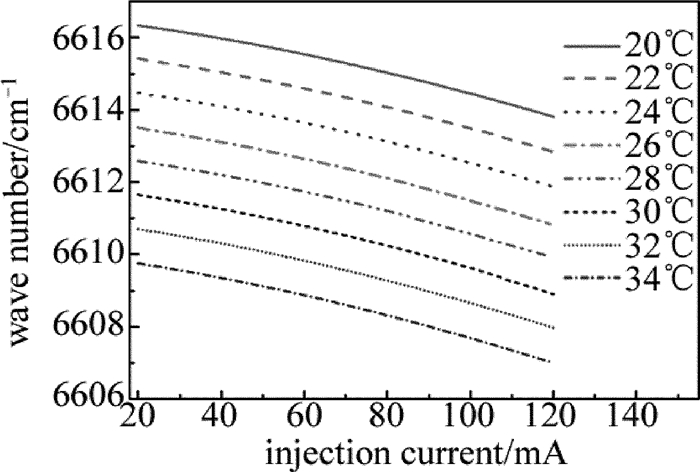
由LCM-6000激光电源设定激光器的工作温度,电压从0mV开始每次增加10mV,通过扫描电压使注入电流从20mA增加到120mA,并记录激光波长随注入电流的变化。图 2是NLK1S5EAAA型DFB半导体激光器在20℃, 22℃, 24℃, 26℃, 28℃, 30℃, 32℃和34℃等几个温度处的激光波长与电流的关系。定标以后,在实验时如果可以确定中心波长,直接将激光器控制器的温度、电流设置成相应数值即可。
因为要测量的NH3是一种无色有刺激性气味的气体,若是气体池气密性欠佳,有NH3漏出会对人体造成伤害,所以可先测量气体池内空气中水的吸收来检验谐振腔调节的是否成功。空气中就有水,故实验条件简单,且吸收峰明显。如果能够明显看出水的吸收峰,说明谐振腔已经调节好,接下来便可以进行NH3气体浓度的测量,并且通过计算发现气体池气密性较好。
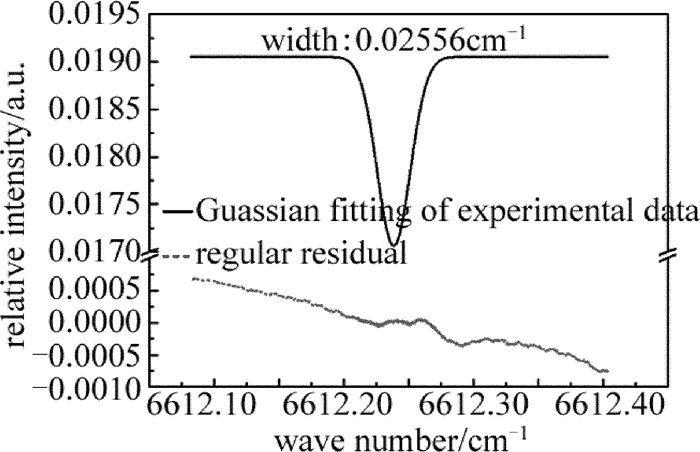
通过查询HITRAN数据库可以知道,NH3在6612.248cm-1处有一个较强的吸收峰,而且在此处没有空气中水汽和CO2的干扰,因此在室温条件下,调节好光路后,将激光器工作的中心波数设置为6612.248cm-1,也就是将1512nm激光器控制器的中心电流设置为85mA,温度设置为25.5℃。图 3所示谱线即是当腔内压强为235Pa时,扫描1000次后的平均谱线。上面实线部分表示的是在6612.248cm-1处NH3分子的Guassian拟合后的腔增强吸收光谱图,下面虚线部分表示的是拟合曲线与实验数据的偏差,即残差图。
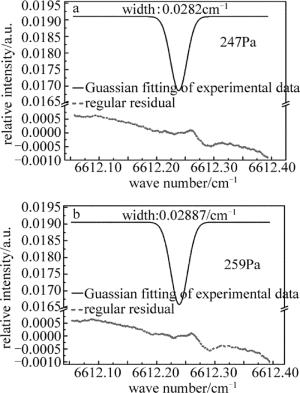
在相同的实验条件下,向气体池内继续充入NH3,通过增加氨气浓度改变池内压强,研究了谱线展宽与气体压强的关系。图 4为腔内压强分别是247Pa, 259Pa时,用Guassian函数对实验数据进行拟合所得的腔增强吸收光谱图。从图中可以看到,当池内气体压力升高,气体谱线宽度也随之加宽。
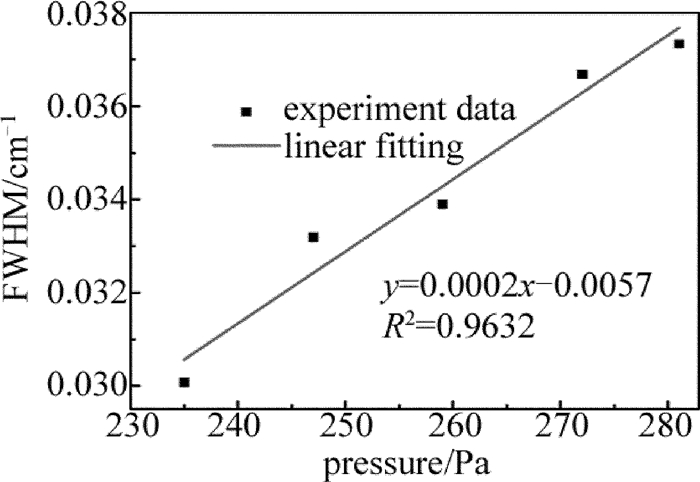
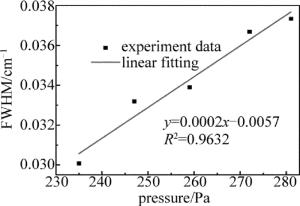
通过增加NH3浓度改变腔内压强,依次测量了腔内压强分别为235Pa,247Pa,259Pa,272Pa,281Pa时NH3在6612.248cm-1处的吸收谱线线宽,并依次读出了每个谱线的半峰全宽(full width at half maximum,FWHM),得到了不同压强下FWHM随气体压强变化的关系曲线,如图 5所示,线性拟合相关系数R2=0.9632,非常接近1。线性拟合相关系数为0.9632,非常接近1。
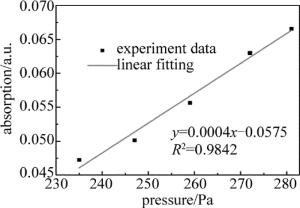
同时,通过数据处理,也分析了改变压强时,吸收度A随腔内压强p的增加的变化情况,得到的关系如图 6所示。在进行数据处理时,通常令:A=-ln(I/I0),从图 6中可以看出,相关系数R2=0.9842,说明A与p之间呈很好的线性关系,符合Beer-Lambert定律,表明腔增强吸收光谱技术可以用于NH3的定量分析研究。
作者对每个压强下的吸收光谱都做了一个数据处理,发现得到的结果之间相差微乎其微,所以,取最小压强下的数据作为代表,计算出此装置能够达到的最小探测灵敏度。图 7a是腔内压强为235Pa时的腔增强吸收光谱图,对实验数据进行Guassian拟合,虚线部分是实验数据曲线,实线部分为高斯拟合曲线,二者的差即为残差,如图 7b所示。对残差噪声部分进行统计分析,若以1个标准偏差为可检测的最小吸收强度,则等噪声探测灵敏度为3.3×10-8cm-1。
-
经过多次重复实验,不断更改各光学元件所处位置,最终成功搭建了腔增强吸收光谱装置,并进行了NH3气体浓度的测量,获得了3.3×10-8cm-1的最小探测灵敏度。实验结果表明:应用高探测灵敏度的腔增强吸收光谱技术,实现了NH3气体浓度的测量,得到了其它光谱技术所不能达到的探测精度。并且因为其在装置、操作、稳定性等各方面性能占有绝对优势,作为大气环境监控和探测领域最主要的方法之一,将有很大的发展空间和广阔的市场前景。

 Map
Map



 DownLoad:
DownLoad: